Crystal Chemistry of a Group of Microporous Inorganic Compounds Obtained Hydrothermally
Belokoneva EL, Stefanovich SY, Dimitrova OV
Belokoneva EL1*,Stefanovich SY2,Dimitrova OV1
1Department of Crystallography and Crystal Chemistry, Faculty of Geology,Moscow State University, Leninskije gory1, 119992 GSP2 Moscow, Russia
2Laboratory of Functional Materials,Chemical Faculty, Moscow State University, Leninskije gory 1, 119992 GSP2 Moscow, Russia
- *Corresponding Author:
- Belokoneva EL
Department of Crystallography and Crystal Chemistry, Faculty of Geology, Moscow State University, Leninskije gory 1, 119992 GSP2 Moscow, Russia
Tel: +7(495)939-4926
E-mail: elbel@geol.msu.ru
Received Date: September 25, 2015 Accepted Date: November 02, 2015 Published Date: November 06, 2015
Abstract
Silicates, phosphates, borates and complex compounds as borosilicates and borophosphates have been synthesized under hydrothermal conditions similar to common in nature, and structurally investigated in the past decade in search of optical nonlinear crystals. Representatives with voids or channels of effective cross sections up to 10 Å are selected and analyzed from the point of view crystal chemistry and possible applications. It is noticed that crystals with framework or layered structures equally accumulate in their voids large ions, or groups of ions, or water molecules from the water solution during the crystallization, and frequently exhibit zeolite properties. Frameworks built on the base of silicate, phosphate or boron tetrahedral demonstrate topological similarity. In a number of crystals the zeolite properties appear in combination with optical non-linearity that is being the bottom of their attraction for integral optics. In non-centro symmetric crystals origin of high optical non-linearity is connected with the highly polarizable electron density of heavy atoms in the voids and channels of the structures. Significant rules are withdrawn from the results of hydrothermal synthesis of crystalline micro porous materials.
Keywords
Hydrothermal synthesis; Microporousstructures; Silicates; Phosphates; Borates; Optical nonlinearity
Introduction
Structures with tetrahedral anionic groups, predominantly with Si- and Al-, condensed via common vertices into frameworks, have been separated from other structure type’s long time ago. Found in the nature, their representatives with special large voids were nominated as zeolites. The most well-known minerals between them are shabazite, cancrinite, fozhazite, analcim, natrolie, harmotome, mordenite, sodalite, ultramarine, and others [1]. Up to now, a huge amount of synthetic zeolites have been synthesized. One of the very first and the most known is “Linde molecular sieve of type A” [1]. Special feature of such structures is sorption of water inside the voids, which may be lost under heating, or substituted by gas molecules of smaller (H2, N2), or larger size (NH4, or C2H5OH, and so on). Thus, dehydrated crystals may be used as sorbent, or ion exchangers for metals separation, or in catalysis. Substances with guest metal atoms are used as luminescence materials, semiconductors or nonlinear crystals in optoelectronics. The latter application results from lack of center of symmetry that occurs in many crystals containing large cavities. Smaller ions in these cavities are frequently displaced from central positions thus producing polar structures with compliant nonlinear activity. Combination of zeolite properties with nonlinear optical activity is of interest in engineering of integrated media for optoelectronic devices [2]. The origin of specific properties is directly connected with size of the voids and their interconnections by pass-ways to produce channels going in one, two or three dimensions.
Principles of systematic and processing of zeolites are summarized in database [3]. Synthesis of the zeolites at present is also carried out using different combination of inorganic and organic components [4]. Good example of the type gives a compound which contains isolated Ga-tetrahedra connected by the organic linker forming porous anionic network. In the material obtained in [4], positively charged ions including alkali-metals and solvent molecules are distributed in a disordered manner in pores, compensating negative charge of the framework. They could be exchanged with different components. Other important and successful example is using layered silicate kanemite NaHSi2O 5 2H2O as a matrix for combination with organic component, namely alkyltrimethyl-ammonium ions [5]. As a result new silicate-organic complex meso porous material was obtained. As far as dimensions of the pores directly determine technical functions of zeolites, crystal structures of them are under intensive investigations on both natural and synthetic representatives. Inorganic compounds with micro porous structures, natural and synthetic, are very numerous, annually increasing in number and often presenting unusual structural features and possessing useful properties. In this paper we do not present comprehensive review of all types of micro porous compounds, but essentially analyze crystal chemistry of some of them recently selected by us among silicates, borosilicates, phosphates, borates obtained in the course in our systematic search for framework nonlinear optical crystals of UV. Their structures with large voids and channels of zeolite character investigated in the last decade (see corresponded references) are compared with the known structure types. Some of them could be used as a matrix for new zeolite materials similar to kanemite mineral. Optical nonlinearity combined with more traditional zeolite properties is of technical importance. In our discussion of structure-properties relations we also focus interest on conditions favorable for hydrothermal synthesis of such the crystals.
Experimental Part
New zeolite-type crystals have been synthesized in our work hydrothermally, similar to many crystals of different classes with tetrahedral anionic groups. The experiments were carried out in a standard autoclaves (volume 5-6 cm3) lined with Teflon. The duration of the experiments (18-20 days) corresponded to the completion of the reaction. The temperature in crystallization process was 250-280°C and the pressure was 70-80 atm. Final cooling after synthesis to the room temperature lasted for 24 hours. We choose the synthesis conditions taking into account known geological processes, therefore, as a rule, initial mixtures of reagents were multi-component. Compounds were crystallized from initial components, oxides and salts with the variation of composition and weight ratio. Boron oxide, phosphorus anhydrite, or silicon oxide exceed was typically used for crystallization of borates, phosphates and silicates correspondingly. Oxides or salts of such metals as Pb, Ba, Sr, RE, Sc, Ga, Fe were employed. The ratio of solid and liquid phase was 1:5. As mineralizers, there were used halogenides or carbonates of alkali ions from lithium to cesium.
Nonlinear optical properties were examined in Second Harmonic Generation (SHG) measurements using Nd:YAG pulsed laser. The SHG experiments were produced on powdered crystals relative to standard α-SiO2 powder with 3-5 mcm crystalline grains. Experimental procedure is principally similar to powder technique of Kurtz and Perry [6] except for our preference for using of thin powders as the most accessible and reliable way for comparison of nonlinear optical activities. Substances with high nonlinearities (classes A and B after Kurtz) on one hand, small nonlinearity (classes C and D, and centrosymmetric E) are usually distinguishable.
More definite conclusions on optical nonlinearity often required more coarse powders. Such powders with grain size up to 100 mcm sometimes are also available in hydrothermal experiments and may be separated in necessary quantities. The X-ray diffraction data were collected at β-filtered Mo Kα radiation with diffractometers Syntex P-1 equipped with point detector, or with diffractometer Xcalibur S equipped with a CCD area detector using graphite-monochromatored Mo Kα radiation. Structure solutions and refinements were made with CSD and SHELXS-97, SHELXL-97 suits of programs. The results of the crystal structures determinations are given in details in corresponding publications including synthesis conditions and properties investigations. Drawing of structures used in crystal chemical analysis is fulfilled using ATOMS suit of programs.
Results and Discussion
Silicates and borosilicates
A new member of the family of porous silicates, K8Gd3Si12O32Cl.2H2O [7], was synthesized and structurally investigated (hereafter Table 1 gives information on unit cell dimensions, space groups, dimensions of voids and nonlinear optical properties of all compounds under investigation). As far as earlier described compounds Na3NdSi6O15.2H2O and K3NdSi6O15 [8] of the same type are ionic-conductive, we consider K8Gd3Si12O32Cl.2H2O as a promising ion-conductor. In this compound it was found for the first time for the whole family that structural channels (Figure 1) are occupied by Cl-ions. Hereafter in the Figures tetrahedra and octahedra are shown; while ions and water molecules are given by circles. Other member of the family investigated recently K7Eu3Si12O32.4H2O [9] is to the most degree close to our silicate, but have more water molecules and no Cl ions in the channels. Large channels surrounded by 8 tetrahedra are preferable for large K-cations, or Cl-ions, or water molecules. Smaller channels formed only by 6 tetrahedra are occupied with RE elements.
| Compound | a, b, c (Å) |
a, b, γ (°) |
Space group | Pores cross section (Å) | Q = I2w / I2wSiO2 |
|---|---|---|---|---|---|
| Silicates and borosilicates | |||||
| K8Gd3Si12O32Cl.2H2O | 6.9060 11.3570 11.6080 |
88.033 88.508 79.377 |
P-1 | 7.0 and 4.6 | |
| K3ScSi3O9.H2O | 13.8797 12.7441 5.7276 |
Pm21n | 7.3 and 6.4 | 0.7 | |
| KNaTmSi8O19.4H2O | 6.5315 6.9935 11.9430 |
90.383 | P2/m | 7.0 and 5.3-3.7 |
|
| K(BSi2)O6 | 10.9320 17.9111 11.0672 |
110.284 | P 21/a | 5.6-2.9 | |
| KBSi2O6 | 4.7126 9.9693 10.4432 |
P 212121 | 5.0 | 1.0 | |
| Pb4.8Na1.2Si8(Si1.2B0.8)O25 | 9.5752 42.565 |
R-3c | 5.5; 3.5 | ||
| Phosphates and borophosphates | |||||
| BaFePO4(OH) | 9.711 8.991 4.912 |
P 212121 | 6.6-2.45 | 1.2 | |
| NaGa3(PO4)2(OH)4 | 11.506 7.270 10.320 |
97.64 | P 21/n | 4.4 | |
| Fe2.5[BP2O7(OH)2][PO3(OH)][PO3 (O0.5OH0.5)]×H2O | 7.3281 9.5762 18.6162 |
101.205 | P 21/n | 9.5-1.5 | |
| Borates | |||||
| Pb2[B5O9](OH).H2O | 11.320 11.440 6.631 |
91.03 | P21/n | 5.5 | |
| Pb2[B5O9]Br | 11.434 11.491 6.538 |
Pnn2 | 5.5 | 80 | |
| Na0.5Pb2[B5O9](OH)0.5×Cl | 11.510 11.470 6.653 |
Pnn2 | 5.5 | 40 | |
| Na0.5Pb2[B5O9](OH)1.5×0.5H2O | 11.426 11.328 6.574 |
Pnn2 | 5.5 | 40 | |
| Ba2[B5O9]Cl×0.5H2O | 11.714 11.552 6.694 |
Pnn2 | 5.5 | 14 | |
| La[B4O6(OH)2]Cl, REE=La-Nd | 6.553 11.256 9.798 |
105.28 | Cc | 2.7 | 30 |
| LnH3[B6O12], Ln = Sm-Lu | 8.385 20.71 |
R3c | 3.75 | 3 | |
| Pb3[B9O16](OH)×B(OH)3 | 10.07 8.530 |
P31c | 9.5 | 20 | |
| Ba3Na[B9O16](OH)×B(OH)4 | 10.253 10.286 8.769 |
90.17 89.80 120.02 |
P1 | 9.5 | 14 |
| Pb6[B12O24]×H2O | 11.432 17.385 |
R-3c | 3.9 | ||
| Pb6(Li0.65Na0.19)[B12O24]I0.84× 0.168H2O | 11.4721 17.2285 |
R-3c | 3.9 | ||
| LaB5O8(OH)2.1.5H2O | 6.447 10.580 12.590 |
89.92 | P 21/n | 10.0-4.7 and 4.7 | |
| La[B4O6(OH)2]Cl | 6.553 11.256 9.798 |
105.28 | Cc | 2.7 | 30 |
| Sr(Na0.4Sr0.1)Na2[B5O8(OH)2](CO3)1-x | 11.322 27.182 6.565 |
94.66 | Bb | 8.7-5.8 and 5.8-4 | 2 |
| LaB5O8(OH)2 | 6.538 10.367 10.47 |
101.58 | P 21/n | 5 and 6.5-3.0 | |
| (Nd0.925Na0.075)Nd[B9O15(OH)2]Cl0.85×2.65 H2O | 10.6987 6.4051 23.1544 |
92.547 | P2/n | 7.7-6.6 and 4.0-2.8 | |
| Sm3[B13O22(OH)3](ÃÆÃÂÃâà ¾ÃÆÃÂÃâÃÂ)·3H2O | 10.7152 6.4188 29.9386 |
98.168 | P2/c | 7.7-4.5 and 4.5-11.3 | |
Table 1: Unit cells parameters, space groups, pores cross section and non-linear optical activities of the new micro porous compounds.
The cross section of the channels is given in Table 1. New micro porous silicate K3ScSi3O9.H2O is a member of the family of rare earth silicates (Sc is the smallest-size analogue of RE elements) [10,11]. The channels in the framework are also constructed with eight polyhedral, six tetrahedra and two octahedra. The corresponding cross sections of the channels are slightly oval being close in dimensions (Table 1). The channels are populated with K ions and water molecules (Figure 2). The smallest ion in the composition, Sc, leads to formation of regular polar framework in contrast to more deformed framework in the case of Ho-silicate. A number of other related frameworks with zeolite properties may be predicted using topology-symmetry principles [10].
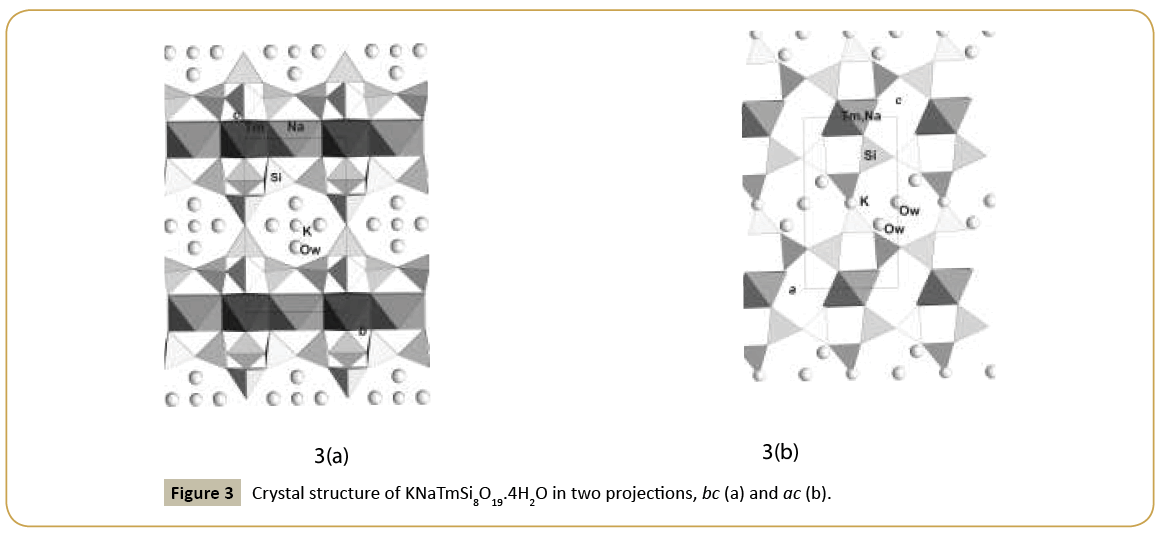
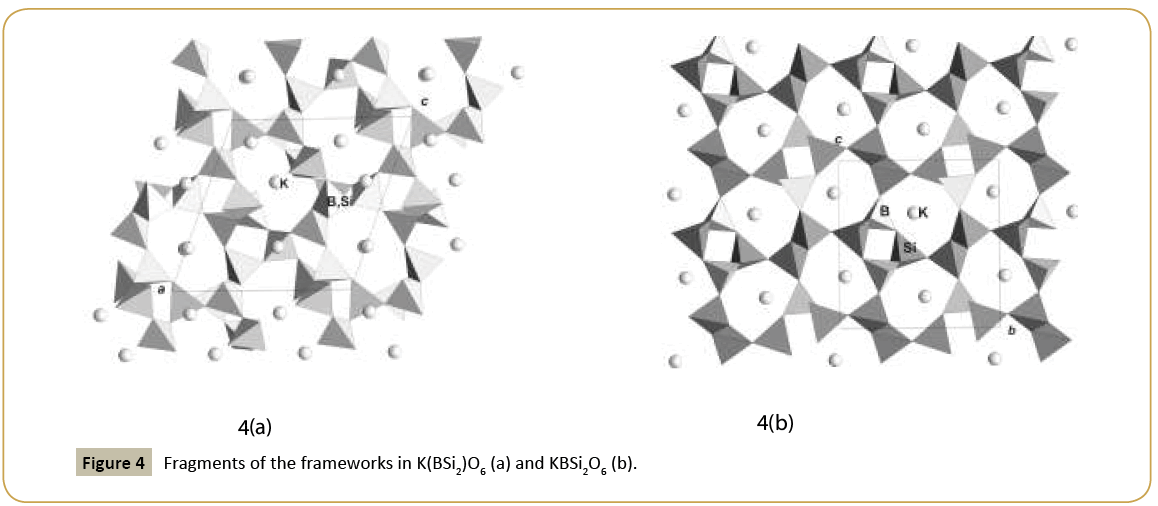
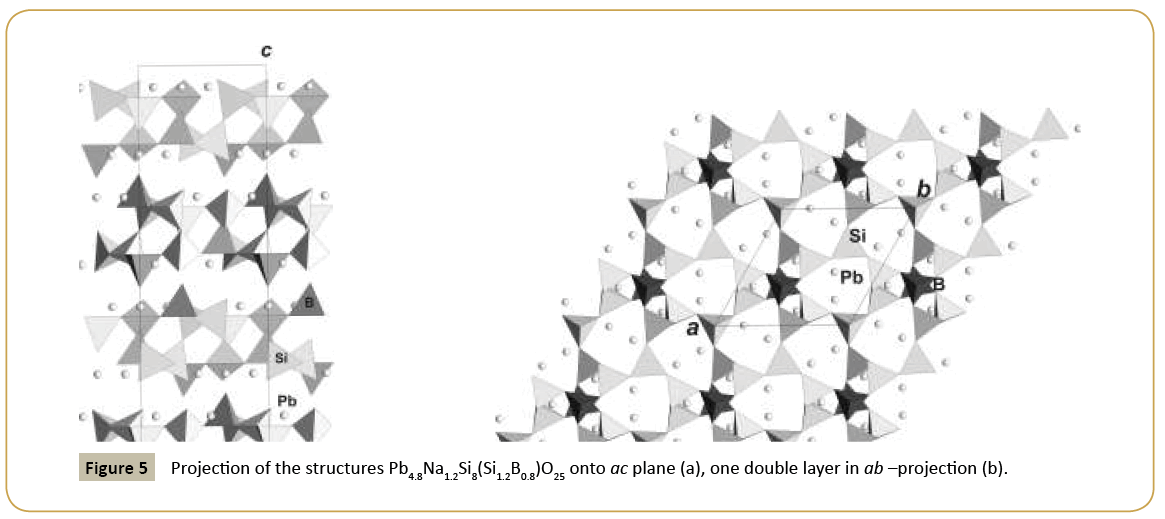
New synthetic silicate KNaTmSi8O19.4H2O belongs to the family of mineral structures as rhodesite, delhayelite, shlikovite, umbrianite, and hilleshaymite and other representatives [12]. It has been shown that all members of the family demonstrate similarity in main structural units of octahedra and tetrahedra and some difference derived in the topology-symmetry analysis. All of them possess large voids occupied by cations, anionic groups and water molecules. Cross sections of large channels with K ions and H2O (Figure 3a and 3b) have close to similar dimensions (Table 1). Layered structures are realized as well in the family (shlykovite, mountainite), being close relative to the framework structures [12], and interlayer space concentrates a lot of ions of different radii and increased amount of water. There is a class of borosilicates with both Si and B tetrahedra presenting simultaneously in the structures. Borosilicates are known as minerals and also as synthetic compounds. Two iso-formula compounds are synthesized: new monoclinic modification of boroleucite K(BSi2)O6 and chiral synthetic KBSi2O6 [13] known also as a rare mineral. They have similar framework structures formed by equal tetrahedra occupied by Si and B with the statistical isomorphic substitution of 1Si:2B in the former compound, while in the latter tetrahedra are occupied in ordered way by Si or B atoms. The voids with 8 and 7 tetrahedral rings accommodate K atoms. On Figure 4a and 4b layered fragments of the frameworks are presented and demonstrate oval voids in K(BSi2)O6 and more symmetrical voids in KBSi2O6 (Table 1). Unusual silicate - formally borosilicate - with the isomorphic impurity of B-atoms in one of the Si-tetrahedral positions, Pb4.8Na1.2Si8(Si1.2B0.8)O25, has a double layer of new type [14]. The layer has relationship with the layer in the structure of benitoite, as well as layers in the structures of lead silicates barisilite and hyttsjoeite (Figure 5a and 5b). Similar topological blocks consisting of an octahedron and six tetrahedra are found in all these structures. New double layer is derived from the hyttsjoeite layer by the replacement of the octahedron in the block by two tetrahedra what leads to the increase of the silicon fraction. Pb ions are located inside oval voids of the layers with the dimensions ~5.6 Å (interlayer space is ~3.5 Å, Table 1) and this structural type and relative minerals are collectors of heavy metals.
Phosphates and borophosphates
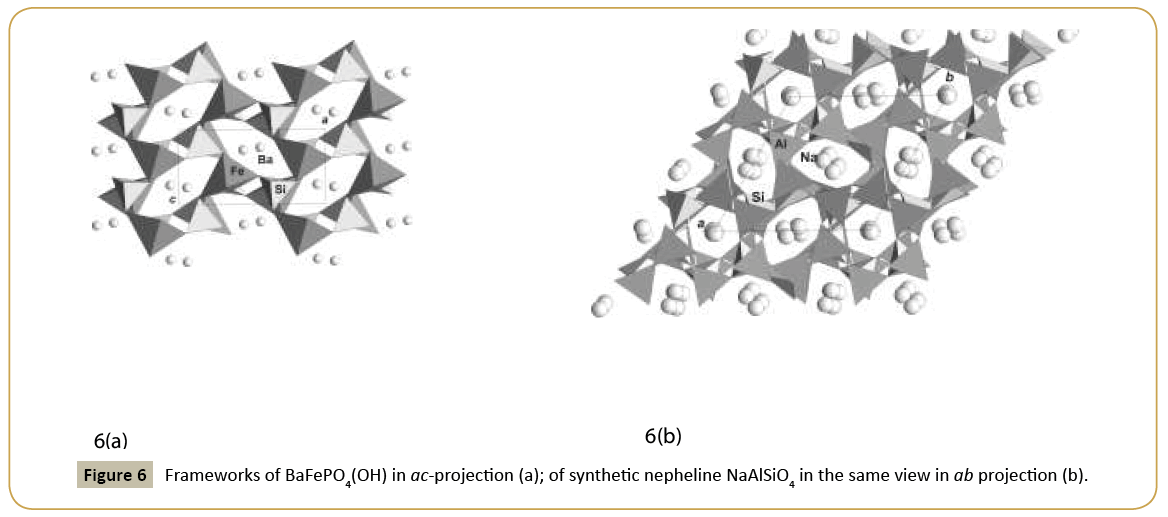
Class of phosphates in our studies gives new examples of framework structures similar to classical zeolite nepheline, NaAlSiO4. Nepheline structure was first solved under assumption of a disorder, but synthetic sample gave good confirmation on real unit cell and framework peculiarities. The analogue between classes with the different tetrahedral groups, silicates or phosphates, is demonstrated on similarity of structures of synthetic nepheline and synthetic BaFePO4(OH) (Figure 6a and 6b) [15]. In nepheline structure, framework is built of Si and Altetrahedra; in Ba-phosphate it is built of P-tetrahedra and Fe-halfoctahedra on tetrahedral position condensed into the framework. Large oval channels are open along b-axes in phosphate and along c-axes in nepheline. They are formed by six tetrahedra (or half-octahedra) with the practically similar dimensions (Table 1) for both structures and are occupied by large Ba ions in new phosphate and by Na-ions in nepheline. Different framework, similar to nepheline, may exist as example not only by pair Fe-P with different ionic radii and coordination, but a pair with smaller radii Si-Al or Si-Be and equal tetrahedral coordination. Thus topology of nepheline type is very stable and may be realized in different representatives. It is favorable for ion exchange and ion conducting properties. Synthetic analogue of mineral brazilianite with Ga substituted of Al in octahedra, NaGa3(PO4)2(OH)4 has a framework structure formed by two types of polyhedra: tetrahedra and octahedra. Open channels along a-axes (Table 1) are occupied by Na ions [16]. Ion exchange is possible in this new microporous phosphate crystal.
New phosphate-borophosphate Fe2.5[BP2O7(OH)2][PO3(OH)] [PO3(O0.5OH0.5)].H2O [17] has microporous framework of Feoctahedra and of several tetrahedra: two isolated P-tetrahedra with the protonated oxygen apexes and one borophosphate sorounit of condensed P- and B-tetrahedra. Large channels which go along a-axis with the cross-section of ~9.5 long and 1.5 Å wide (Table 1) are occupied by water molecules.
Borates
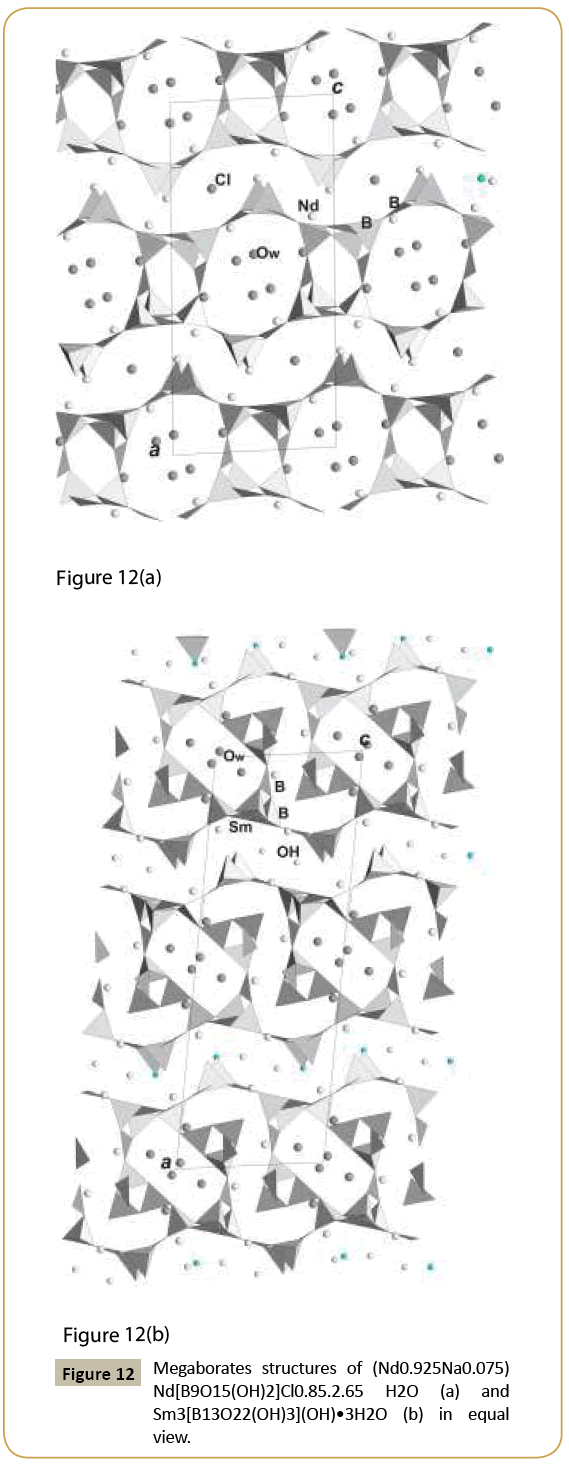
Borates are known as indispensable in many applications: nonlinear optical, luminescence, magnetic. Their ion exchanging or ions conducting properties Figure 2 Projection of the structure K are also very interesting. In class of borates we managed to reveal one of the most intriguing structures, namely, noncentrosymmetric hilgardites: their name being after rare mineral hilgardite, Ca2B5O9Cl.H2O. All representatives of the family have different compositions and frameworks of different symmetry, but nevertheless they are close to each other. The framework is composed of B-tetrahedra and B-triangles combined in blocks, chains, than into equal layers joint into frameworks by different symmetrical combinations, what was analyzed in [18]. Polar-structure of natural hilgardite, sp. group Aa, has never been obtained as synthetic phase but only as its polar topologically new modification [19]. Centrosymmetric variants are represented with Pb2B5O9(OH).H2O [18] and Na0.5Ba2B5O9FCl0.5 [20]. Most promising for application are orthorhombic polar modifications, known for many compositions: Ca2B5O9Br [21], Eu2B5O9Br [22], Pb2B5O9Br [23], Na0.5Pb2B5O9(OH)1.5.0.5H2O, (Figure 7) [24], Na0.5Pb2B5O9Cl(OH)0.5 [25], Ba2B5O9Cl.O.5H2O [26], Ba2B5O9Cl [27], Pb2B5O9Cl [28], Pb2B5O9I [29]. Chemical formulas and structure investigations demonstrate that in the similar framework open channels may be filled with different cations, anions, hydroxyl groups and water molecules. Nonlinear optical properties were established in polar crystals. The largest SHG response among borates was found in Pb2B5O9I: 13.5 times that of KDP and ~3 time that of Pb2B5O9Br according to [29]. Relative to α-SiO2, nonlinear optical activity of different hilgardite representatives varies from maximal in Pb2B5O9I and Pb2B5O9Br to middle in Na0.5Pb2B5O9Cl(OH)0.5 and Na0.5Pb2B5O9(OH)1.5.0.5H2O, and to lower in Ba2B5O9Cl.0.5H2O (Table 1). It was shown [23] that the optical nonlinearity regularly increases in series of hilgardites upon the substitution of the cations Ca → Sr → Ba → Pb and the anions Cl → Br. This suggests that the role played by the extra-framework atoms in the voids, as well as electron density on their bonds, is the most important in the formation of nonlinearity in compounds with the similar boron–oxygen framework. Large cavities going along c-axis are real open channel. Its cross section has dimension ~5.5 Å for all modification of microporous structures. Complicate compositions for different representatives display possibility for absorption of ions from the solution and principally to the ion exchange for this structure type often combined with the optical nonlinearity. Trigonal polar borates (Figure 8) with common formula REE[B6O9(OH)3], REE=Sm – Lu, are synthesized, structurally investigated and analyzed from crystal chemistry point of view in [30]. These compounds demonstrate structural relation to cubic (natural or synthetic) boracites. Many non-cubic boracites are also very close to Li-boracite Li4[B7O12]Cl known to possess Li-ion conductivity [31]. The framework of this type of borates is constructed from six member rings of triangles and tetrahedra, however it has common features with that of berillonite and nepheline minerals which are built only from BO4-tetrahedra. Compared to Li-boracite, RE metals occupy Cl positions - central in the open channels (Table 1). Electric conductivity found in some representatives of REE[B6O9(OH)3 family exceeds 10-6 S/ cm. and may be explained by proton diffusion (H-position corresponds not to OH-group but to H+ as cation). Except that, crystals demonstrate nonlinear optical properties (Table 1). New borates Pb3(OH)B9O16.B(OH)3, (Pb0.5??0.5)3(OH)B9O16.B(OH)3 and ??3Na(OH)B9O16. B(OH)4 [32] have framework of borate nongroup built of six polar tetrahedra added by three polar triangles. Large open channels along c-axis have cross-section of ~8.75Å. Two variants of channels occupations are found with B(OH)3 triangles (full sassoline molecule) (Figure 9) and with B(OH)4- tetrahedra on the same position at the center. Large cations as Pb, Ba are closer to the walls of the open channels along c-axis as in hilgardite and may isomorphically substitute each other and that demonstrates ions exchange properties. Local polar environments are aligned in the structure and polar material is observed. Concern structure-properties relation, it was concluded [32] that the same rules are valid for nona-borates family as for hilgardite family in the rows with the different ions. The observed maximal signal relative to α-SiO2 (Table 1) is for Pb-nonaborate with the most polarized large Pb ion; it is somewhat weaker for borate with Ba and Na atoms and polar distorted B-tetrahedral groups, incorporated into channels of the framework; and it is the weakest for borate with isomorphic substitution of Pb, Ba. Even in the last case, the nonlinear optical activity of the borate with the filled framework is compared well with the nonlinear activity of the BBO - β-BaB2O4 borate. This indicates once again that borates with filled frameworks are more promising materials for attaining a maximum optical nonlinearity as compared to borates with isolated polyanion groups. Unusual dodecaborate ring is found in structures of Pb6[B12O24].H2O and Pb6(Li0.65Na0.19) [B12O24]I0.84. 0.168H2O obtained in our hydrothermal synthetic experiments [33]. Open channels here with cross-section ~3.9 Å are centered on complex ring and populated with Li(Na), I-ions and water molecules taken from the solution during crystallization. Dodecaborate structures have similarity with beryl and cordierite minerals [33]. The last mineral contains CO2-impurity in the voids of open channels; it is known as sorbent and filter for motor-cars. New borate LaB5O8(OH)2.1.5H2O [34] has a framework with large oval channels (Table 1) occupied by water molecules; La-cations are also inside the channels in the holes of corrugated layers (Figure 10). Other smaller voids are empty. Layers of different topology and symmetry are very typical anionic radicals for borates. New synthetic polar borates REE[B4O6(OH)2]Cl, REE=La- Nd [35] have layered structure with the rare earth and chlorine ions in the interlayer space, and it is good nonlinear optical crystal (Table 1). The example of structure with the layer relative to hilgardite is rare carbonatoborate Sr(Na0.4Sr0.1)Na2[B5O8(OH)2] (CO3)1-x [36], Figure 10. The interlayer space is filled by ions, water molecules or CO3-triangles which most real hinder condensation of layers into framework. Similar compound was found also for Ca-representative. Other new layer in new borate LaB5O8(OH)2 [37] is strongly corrugated and curved, and in the holes of its walls are La ions. New examples of borates with structures possessing large voids inside the complex layers and interlayer space are megaborates (Nd0.925Na0.075)Nd[B9O15(OH)2]Cl0.85.2.65H2O [38] and Sm[B13O22(OH)3](OH).3H2O [39] (Figure 12). Double layer of Nd-borate has similar topology with the framework fragment of LaB5O8(OH)2.1.5H2O with the two types of voids: larger and smaller (Table 1). The former is occupied by Na, Cl ions and water molecules, the latter is also empty. Cations, anions and (OH)- groups are in the inter layer space (Figure 12). Sm-borate has also double layer derived from Nd-layer (Figure 12). It contains extension in a form of triborate circle with the additional triangle. Because of that configuration of voids is changed: S-form voids are occupied by Sm ions whereas larger contain water molecules. Wide inter layer space accommodates (OH)-groups and Sm ions in the holes of corrugated layers with the similar cross section as in Nd-borate.
Conclusions
New silicates, borosilicates, phosphates, borophosphates and borates with microporous structures are synthesized in hydrothermal conditions. Significant rules are withdrawn from the results of hydrothermal synthesis of crystalline microporous materials. For borates, excess of boron anhydrite in ratio REE/ B2O3 leads to complexity and higher degree of anionic radical condensation of borate anionic units from the layer to the framework; higher mineralizer’s concentration up to 20% favors participation of alkali metals or halogen-ions in the structures. As example, presence of NaBr gives sodium containing hilgardites; presence of ions Cl- and Br- gives Na0.5Pb2B5O9Cl(OH)0.5, Ba2B5O9Cl.0.5H2O and Pb2B5O9Br etc. Despite Ba and Pb ions are chemically very different, similar nonaborates with both large cations were obtained in our experiments. Phosphate systems demonstrate important role of presence of B2O3 up to 10% as buffer, and that allows to correct pH of the solution and to slow down crystallization process what assist growth of crystals with a structures with large voids. That was realized for Ga-brazilianite and Ba, Fe phosphate with the structure similar to nepheline. Participation of B2O3 as mineralizer was also detected for the silicate systems with REE ions as a key to forming high condensed silicate anionic radicals. Phosphates and silicates frequently produce structures with large voids mainly at high temperatures of synthesis, nevertheless presence of B2O3 make possible crystallization of such structures at lover temperatures. There is no principal difference between tetrahedral frameworks of compounds which formally belong to different classes: silicates, phosphates or borates. Potentially, all of them may include ion-conducting, ion-exchanging and optical non-linear crystals. Combination of these properties in one and the same material may be of technical importance. To meet the requirement in microporous structures, dimensions of pores characterized by cross sections from ~4 to 10 Å, specifies the crystal chemistry prerequisite. A number of crystal structures possess large voids or interlayer spaces where cations, anions, hydroxyl groups and water molecules are located. From the point of view of ionexchanging and optical nonlinear properties there is no principal difference between crystals with frameworks or layers. All of them demonstrate zeolite character and possess voids or cavities occupied by different components taken from the solution during crystallization, and all of them may be principally considered as "breathing" and controlling their optical non-linearity. Borates with filled frameworks are much more promising materials for attaining a maximum optical nonlinearity as compared to borates with isolated polyanion groups.
Acknowledgments
This study was supported by the RFBR Grant No. 14-03-00480a.
References
- Bragg L, Claringbull GF, Taylor WH (1965) Crystal structures of minerals. G. Bell and Sons Ltd., London.
- Serra-Crespo P, Van der Veen MA, E, Filinchuk, et al. (2012) NH 2-MIL-53 (Al): A high contrast reversible solid state nonlinear optical switch. J. Am. Chem. Soc., 134: 8314–8317.
- Baerlocher C, McCusker LB,Database of Zeolite Structures.
- Banerjee D, Kim SJ, Wu H, Xu W, Borkowski LA, Li J, Parise JB (2011) Anionic Gallium-Based Metal-Organic Framework and Its Sorptiom and Ion-Exchange Properties. Inorg Chem 50: 208-212.
- Inagaki S, Fukushima Y, Kuroda K (1993) Synthesis of highly ordered mesoporous materials from layered polysilicate. J Chem Soc Chem Commun 680-682.
- Kurtz SK, Perry TT (1968) A powder technique for the evaluation of nonlinear optical materials. J Appl Phys 39: 3798-3812.
- Zorina AP, Belokoneva EL, Dimitrova OV (2014) K8Gd3Si12O32Cl.2H2O, a new member of family of porous silicates with K and RE elements. Cryst Reports 59: 36-40.
- Haile SM, Wuensch BJ (2000) Structure, phase transition and ionic conductivity of K3NdSi6O15xH2O II Structure of β-K3NdSi6O15. Acta Cryst B56: 349-354.
- Ananias D, Kostova M, Paz FAA, Neto ANC, De Moura RT, (2009) Molecule-like Eu 3+ dimmers embedded in an extended system exhibit unique photoluminescence properties. J Am Chem Soc 24: 8620-8624.
- Belokoneva EL, Zorina AP, Dimitrova OV (2013). New framework hydrous silicate K3ScSi3O9.H2O related to the high - temperature anhydrous silicate K3HoSi3O3 and symmetry analysis of a phase transition with prediction of structures. Cryst Reports 58: 586-593.
- Ponomarev VI, Philipenko OS, Atovmjan LO (1988) Crystal structures of K,Ho-triorthosilicate K3HoSi3O9(OH)2 at 300 K and its de-hydration product K3HoSi3O9. at 880 K. Russ Phys Crystallogr 33: 98-102.
- Belokoneva EL, Topnikova AN, Dimitrova OV, Volkov AS (2014) KNa3TmSi8O19.4H2O, a new layered silicate related to rhodesite-shlykovite-delhayelite-umbrianite-guenterblassite-hilleshaymite: topology-symmetry analysis of the OD-family and structure prediction. Cryst Reports 59: 513-522.
- Belokoneva EL, Dimitrova OV, Stefanovich S (2010) Synthesis and crystal structure of a new orthophosphate with a mixed framework related to alumosilicate of nepheline group. Cryst Reports 55: 575-582.
- Belokoneva EL, Dimitrova OV (2011) Crystal structure (Pb4.8Na1.2)Si8(Si1.2B0.8)O25 with new double tetrahedral layer and its comparison with hyttsjoeite, barisilite, benitoite and langasite. Cryst Reports 56: 110–116.
- Belokoneva EL, Dimitrova OV (2010) Synthesis and crystal structure of a new orthophosphate with a mixed framework related to alumosilicate of nepheline group. Cryst Reports 55: 216-220.
- Ruchkina AA, Belokoneva EL, Dimitrova OV (2003) Synthesis and crystal structure of gallium analogue of brazilianite NaGa3[PO4]2(OH)4. Russ J Inorg Chem 48: 632-635.
- Belokoneva EL, Dimitrova OV (2015) Fe2.5[BP2O7(OH)2][PO3(OH)].[PO3(O0.5OH0.5)]×H2O – new phosphate – borophosphate with the microporous structure. Cryst Reports 60: 361-366.
- Belokoneva EL, Dimitrova OV, Korchemkina TA (1998) Pb2B5O9(OH).H2O: a new centrosymmetric modification of natural hilgardite, The hilgardite group of structures as members of the OD-family. Cryst Reports 43: 810-819.
- Wei Q, Cheng JW, He C, Yang GY (2014) An acentric calcium borate Ca2[B5O9].OH.H2O: synthesis, structure and nonlinear optical property. Inorg Chem 53: 11757-11763.
- Yu H, Pan S, Wu H, Yang Z, Dong L (2013) Effect of rigid units on the symmetry of the framework: design and synthesis of centrosymmetric NaBa4(B5O9)2F2Cl, and noncentrosymmetric NaBa4(AlB4O9)2Br. Crystal Growth and design 13: 3514-3521.
- Lloyd PJ, Levasseur A, Fouassier C (1973) Structure cristalline du bromoborate Ca2B5O9Br. J Sol State Chem 6: 179-186.
- Machida KJ, Adachi GY, Yasuoka N, Kasai N, Shiokawa J (1980) Crystal structure of europium (II) bromoborate. Inorg Chem 19: 3807-3811.
- Belokoneva EL, Kabalov K, Dimitrova OV, Stefanovich S (2002) New polyborate with the high optical nonlinearity Pb2[B5O9]Br of hilgardite group. Cryst Reports 47: 1013-1017.
- Al Ama AG, Belokoneva EL, Stefanovich S, Dimitrova OV, Mochenova NN (2006) A new nonlinear optical borate Na0.5Pb2B5O9(OH).0.5H2O from the orthorhombic hilgardite family. Cryst Reports 51: 395-400.
- Belokoneva EL, Korchemkina TA, Dimitrova OV, Stefanovich S (2000) Na0.5Pb2B5O9Cl(OH)0.5 - a new polar variety of hilgardite containing Na+ cations in the cavities af the framework. The OD-family of the 5:2D+3T pentaborates: hilgardites, heidornite, probertite and ulexite. Cryst Reports 45 : 744-753.
- Ferro O, Vinogradova SA, Pushcharovsky D, Dimitrova OV (2000)Crystal structures of two new Ba borates pentaborate, Ba2[B5O9].O.5H2O and Ba2[B5O9(OH)](OH). J All Comp 305: 63-71.
- Held P, Liebertz J, Bohaty L (2002) Crystal structure of dibarium pentaborate chloride, Ba2B5O9Cl. Z Krist NCS 217 (2002) 463-464.
- Egorova BV, Olenev AV, Berdonosov PS, Kuznetsov AN, Stefanovich SY, et al. (2008) Lead-strontium borate halides with hilgardite structure type and their SHG properties. J Sol State Chem 181: 1891-1898.
- Huang YZ, Wu LM, Wu XT, Li LH, Chen L, et al. (2010) Pb2B5O9I an iodite borate with strong second harmonic generation. J Am Chem Soc 132: 12788-12789.
- Belokoneva EL, Ivanova AG, Stefanovich SY, Dimitrova OV, Kurazhkovskaya VS (2004) New Ln(B6O9(OH) borates (LN=Sm-Lu): structure, properties and structural relation to cationic conductors (Li-boracites). Cryst Reports 49: 603-612.
- Jeitschko W, Bither TA, Bierstedt PE (1977) Crystal structure and ionic conductivity of Li boracites. Acta Cryst B33: 2767-2772.
- Belokoneva EL, Stefanovich SY, Dimitrova OV, Mochenova NN, Zubkova NV (2009) Structure and nonlinear optical properties of the family of lead and barium nonaborates with a zeolite-like framework. Cryst Reports 54: 814-821.
- Belokoneva EL, Derkach IK, Dimitrova OV (2013) Crystal structure of new variety of lead dodecaborate Pb6(Li0.65Na0.19) [B12O24]I0.84·0.168H2O. Cryst Reports 58: 404-409.
- Ivanova AG, Belokoneva EL, Dimitrova OV, Mocheniva NN (2006) New borate LaB5O8(OH)2·1.5H2O with a complex framework, its place in the structural system based on symmetry and topology analysis in terms of the OD theory. Russ J Inorg Chem 51: 862-868.
- Belokoneva EL, Stefanovich SY, Dimitrova OV., A.G.Ivanova (2002) New nonlinear optical crystal of Ln[B4O6(OH)2]Cl (Ln=Pr, Nd) and teir structural relation to pentaborats in terms of the OD-theory. Russ J Inorg Chem 47: 317–323
- Belokoneva EL, Dimitrova OV, Mocheniva NN (2009) Synthetic Na,Sr-carbonatoborate with a new type of pentaborate layer: the OD-nature of the structure and its correlation with volkovskite, veatchite, and hilgardites. Cryst Reports 54: 6–12.
- Ivanova AG, Belokoneva EL, Dimitrova OV, Mocheniva NN (2006) Compound La[B5O8(OH)2] with a new type of pentaborate layer based on the 5[3T+ 2D] block: topology–symmetry analysis and the position in the structural system. Cryst Reports 51: 584–588.
- Belokoneva EL, Shagivaleeva IK, Dimitrova OV, Mocheniva NN (2010) New layer borate (Nd0.925Na0.075)Nd [B9O15(OH)2]Cl0.85·2.65H2O and its place in the structural systematic. Cryst Reports 55:753-759.
- Belokoneva EL, Zorina AP, Dimitrova OV (2012) New layer megaborate Sm3[B13O22(OH)3](OH) ·3H2O and its place in the structural systematic. Cryst Reports 57:499-504.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences