The Structural Study of [2-Cl-C6H4C(O)NH] P(O)[NHC6H4-4-CH3]2
Pourayoubi M, Taherzadeh M, Dusek M and Kucerakova M
Taherzadeh M1, Pourayoubi M1*, Dusek M2 and Kucerakova M2
1Department of Chemistry, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad, Iran
2Institute of Physics of the Czech Academy of Sciences, Na Slovance 2, 182 21 Prague 8, Czech Republic
- *Corresponding Author:
- Pourayoubi M
Department of Chemistry, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad, Iran
Tel: +989155143058
E-mail: chebhamouda@yahoo.fr
Received date: October 17, 2016; Accepted date: November 22, 2016; Published date: November 30, 2016
Citation: Taherzadeh M, Pourayoubi M, Dusek M, et al. The Structural Study of [2-Cl-C6H4C(O)NH] P(O)[NHC6H4-4-CH3]2. Struct Chem Crystallogr Commun. 2016, 2:2.
Abstract
A new phosphoric triamide with the formula [2-Cl-C6H4C(O)NH]P(O)[NHC6H4-4- CH3]2 has been investigated by spectroscopic methods and X-ray crystallography. This compound crystallizes in the monoclinic system, with P21/c space group. In this molecule, the P atom has a distorted tetrahedral environment. The N atoms bonded to P atom have mainly sp2 character. In the crystal, the molecules are aggregated through NCP―H…Oâ•ÂÂP and NP―H…Oâ•ÂÂC hydrogen bonds in a linear arrangement along the b axis, by forming a sequence of alternate R22 (8) and R22 (12) motifs (NCP is the nitrogen atom of C(O)NHP(O) segment and the NP stands the two other nitrogen atoms bonded to the P atom). Furthermore, C―H…O and C―H…Cl intermolecular interactions complete a 3D structure.
Keywords
Phosphoric triamide; Hydrogen bond; Crystal structure; Graph set motsssif
Introduction
Phosphoramides constitute a well-studied sub-class of phosphorus(V)-nitrogen compounds due to the biological activity of some derivatives and growing applications in pharmacological and agricultural industry [1-3]. These compounds can bind to a metal cation as an oxygen-donor ligand [4-6]. Within this sub-class, compounds with a P(O)[NHC(O)][N]2 skeleton are interesting for the preparation of chelating bidentate ligands and conformational studies of the C(O)ÃÆâÃâââ¬Ãâââ¬Â¢NHÃÆâÃâââ¬Ãâââ¬Â¢P(O) fragment [7-12]. Here we report the synthesis, spectroscopic characterizations, and X-ray crystallography of N-(2-chlorobenzoyl)-N’, N"-bis(4- methylphenyl)-phosphoric triamide (C21H21ClN3O2P). The possibility of forming different hydrogen bonds is also discussed.
Experimental
X-ray crystallography
A single crystal of the investigated compound was measured with a SuperNova four-circle diffractometer of Rigaku Oxford Diffraction equipped with a 40W micro focus CuKα X-ray source collimated by mirrors, and CCD detector Atlas S2. Measurement was done at 95K using a Cryostream 800 Plus chiller. Measurement and data processing of the CCD images were done by program CrysAlisPro [13], structure was solved by Superflip [14] and refined by Jana 2006 [15]. All atoms except hydrogen were refined anisotropically. Hydrogen atoms belonging to carbon were kept at the expected positions while positions of hydrogen atoms belonging to nitrogen were refined using a restraint on N-H distances keeping them the same. Isotropic ADP of hydrogen atoms were constrained to 1.2 multiple of the Ueq of their parent atom. No unusual features were found during the structure solution and refinement.
Spectroscopic measurements
1H, 13C and 31P NMR spectra were recorded on a Bruker Avance III-300 spectrometer. 1H and 13C NMR spectra were referenced using the solvent CDCl3 resonances (7.29 and 77.07 ppm for 1H and 13C, respectively) for [2-Cl-C6H4C(O)NH]P(O)[Cl]2 and using DMSO-d6 resonances (2.50 ppm,1H, and 39.52 ppm,13C) for [2-Cl- C6H4C(O)NH]P(O)[NHC6H4-4-CH3]2. The 31P NMR spectra were calibrated using the “absolute referencing” from the related 1H spectra. IR spectra were recorded on a Thermo Nicolet Avatar 370 FTIR spectrometer and on a Buck 500 scientific spectrometer using KBr discs. The mass spectra were recorded with an MS model of the CH7A Varian Detector. Elemental analyses (C, H and N) were performed on a Thermo Finnigan Flash 1112EA elemental analyzer and the melting points were recorded with an Electrothermal IA 9000 apparatus.
Synthesis
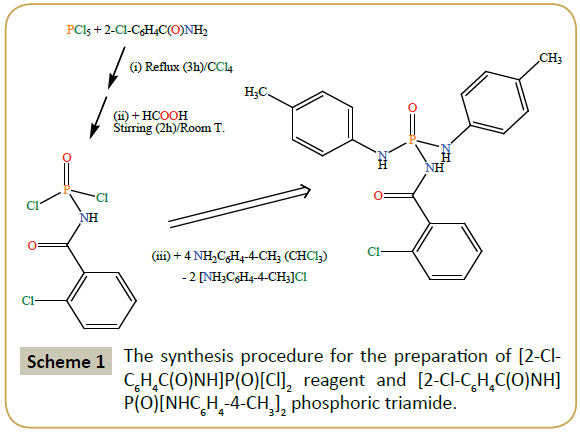
Caution: Phosphorus pentachloride is very sensitive to the moisture and its partial hydrolysis gives phosphoryl trichloride, so, for the preparation of the [2-Cl-C6H4C(O)NH]P(O)[Cl]2 reagent, dry CCl4 solvent was used. Carbon tetrachloride was dried over P2O5 under reflux condition and distilled prior to use. A previous article mentioned the synthesis of [2-Cl-C6H4C(O)NH]P(O)[Cl]2 reagent and its melting point (92–93°C) [16]. The procedure reported here is similar to the above-mentioned literature method with a few modifications. The modifications consist in replacing the dry C6H6 solvent by dry CCl4 and also using PCl5 as phosphorus-chlorine reagent instead of 2-Cl-C6H4C(O)NÃÆâÃâââ¬Â¢ÃâÃÂPCl3. A schematic protocol for the synthesis of [2-Cl-C6H4C(O)NH] P(O)[Cl]2 reagent and [2-Cl-C6H4C(O)NH]P(O)[NHC6H4-4-CH3]2 phosphoric triamide is given in Scheme 1. It should be noted that the 2-Cl-C6H4C(O)NÃÆâÃâââ¬Â¢ÃâÃÂPCl3 reagent is also formed during the procedure used in this paper when PCl5 and 2-Cl-C6H4C(O)NH2 react (stage (i) in Scheme); however, to avoid the hydrolysis of sensitive 2-Cl-C6H4C(O)NÃÆâÃâââ¬Â¢ÃâÃÂPCl3 reagent, we preferred to continue the reaction with adding HCOOH to the solution containing 2-Cl- C6H4C(O)NÃÆâÃâââ¬Â¢ÃâÃÂPCl3 (stage (ii) in Scheme). We further studied the [2-Cl-C6H4C(O)NH]P(O)[Cl]2 reagent with IR and NMR experiments and mass spectrometry. Moreover, the fusion point measured by us is a few more than that was reported in literature, probably due to different crystallinities of product obtained in different solvents or the more purity of sensitive reagent obtained by us.
Synthesis of [2-Cl-C6H4C(0)NH]P(0)[Cl]2
The [2-Cl-C6H4C(O)NH]P(O)[Cl]2 reagent was prepared by reflux of phosphorus pentachloride and 2-chlorobenzamide in equimolar ratio in dry CCl4 for 3 h (stage (i) in Scheme). The completion of this stage was monitored by stopping of the evolution of gas bubbles in an oil vessel. The reaction mixture was then cooled at an icebath and formic acid with similar ratio of the noted reactants was syringed drop-wise into the cold solution, and resulting colorless solution was stirred at 25°C for 2 h. After completion of the reaction, the stirring was stopped and the solvent was removed at a reduced pressure (stage (ii) in Scheme) to form a solid white product (yield: above 80%). Fusion point: 99°C. IR (cm–1): 3091, 2834, 2676, 2553, 1712, 1673, 1592, 1477, 1431, 1405, 1292, 1264, 1227, 1162, 1105, 1046, 960, 901, 873, 776, 752, 716, 653, 592, 561, 509, 465, 405. 31P{1H}NMR (acetone-d6): δ=1.78 (s). 1HNMR (CDCl3): δ=7.41 (m, 1H), 7.50 (m, 2H), 7.74 (m, 1H), 9.62 (d2J(P,H)=8.4 Hz, 1H, NH). 13CNMR (CDCl3): δ=127.34, 130.51, 130.89,131.67, 131.80 (d3J(P,C)=10.4 Hz), 133.34, 165.64 (d2J(P,C)=3.6 Hz). MS (70 eV, EI): m/z (%)=238 (30) [M–35Cl]+ (M is based on two 35Cl and one 37Cl), 139 (88) [C7H4 35ClO]+, 138 (100) [C7H5 35ClN]+, 101 (63) [P35Cl2]+, 75 (95) [CH2PNO]+, 47 (56) [PO]+. (C7H5Cl3NO2P) (%): C=30.83; H=1.84; N=5.14; found: C=30.83; H=1.72; N=5.21.
Synthesis of [2-Cl-C6H4C(O)NH]P(O)[NHC6H4-4-CH3]2
A solution of p-toluidine (10 mmol) in CHCl3 (25 ml) was added dropwise to a solution of [2-Cl-C6H4C(O)NH]P(O)[Cl]2 (2.5 mmol) in the same solvent (25 ml) at 273 K. After 4 h of stirring at an ice bath temperature, the process was stopped and the mixture kept at room temperature for a few days to remove the solvent. The solid obtained was washed with distilled water to remove the [NH3C6H4(4-CH3)]Cl salt (stage (iii) in Scheme). Suitable single crystals were obtained from a solution of the synthesized compound in CHCl3/CH3CN (1:4 v/v) at room temperature after a few days (yield: above 80%). Fusion point: 242°C. IR (cm–1): 3336, 3288, 3081, 2889, 1670, 1517, 1446, 1385, 1289, 1216, 945, 821, 740. 31P{1H}NMR (DMSO-d6): δ=–6.17 (s). 1HNMR (DMSO-d6): δ=2.22 (s, 6H, Me), 7.02 (d, 3J(H,H)=8.4 Hz, 4H), 7.08 (d3J(H,H)=8.7 Hz, 4H), 7.36 (m, 2H), 7.45 (m, 2H), 7.74 (d2J(P,H)=9.9 Hz, 2H, NH), 10.05 (s, 1H, NH). 13CNMR (DMSO-d6): δ=20.71, 127.33, 129.78, 131.76, 136.34 (d, 3J(P,C)=9.1 Hz), 138.89, 168.35. MS (70 eV, EI): m/z (%)=415 (8) [M]+ (37Cl), 413 (40) [M]+ (35Cl), 412 (43) [M –1]+, 138 (65) [2-35Cl-C6H4CNH]+, 137 (20) [2-35Cl-C6H4CN]+, 107 (50) [C7H8NH]+. (C21H21ClN3O2P) (%): C=60.89; H=5.07; N=10.15; found: C=60.53; H=5.14; N=10.39.
Description of the crystal structure
The asymmetric unit of [2-Cl-C6H4C(O)NH]P(O)[NHC6H4-4-CH3]2 consists of one molecule, as shown in Figure 1. The crystal data and refinement parameters are listed in Table 1 and selected bond distances and angles are listed in Table 2. The P atom has a distorted tetrahedral configuration as has been noted for other phosphoric triamides [17], with bond angles around the P atom in the range of 99.54(5)–118.89(6)°. All P—N bonds in this compound are shorter than a typical phosphorus-nitrogen single bond (1.77 Å) and longer than a typical phosphorusnitrogen double bond (1.57 Å) [18], caused probably by the overlap of the electrostatic effects of the P—N polar bonds with their corresponding sigma bonds [19]. The PÃÆâÃâââ¬Ãâââ¬Â¢NP distances of 1.6335(11) and 1.6465(11) Å are significantly shorter than the related PÃÆâÃâââ¬Ãâââ¬Â¢NCP bond distance (1.6862(11) Å), resulting from the electronic effect caused by the C(O) group. This prolongation can also be found in the CSD for different types of PÃÆâÃâââ¬Ãâââ¬Â¢N bonds in C(O)NHP(O)-based phosphoric triamides [20]. The phosphoryl and carbonyl groups, separated by the NH unit, adopt an antiposition with respect to each other, which is in agreement with previously reported acyclic phosphoric triamide compounds containing a C(O)NHP(O)(NH)2 skeleton [21,22]. The PÃÆâÃâââ¬Â¢ÃâÃÂO bond length is of standard value (1.4770(9) Å), and the bond-angle sums of about 355° for one nitrogen atom and about 360° for two other nitrogen atoms (P—N—C+C—N—H+H—N—P) confirm their sp2 character. The criteria for distinguishing between planar and non-planar geometries from bond-angle sums are the same as previously proposed: N(planar) and N(pyramidal) refer to the cases with Σ ≥ 352.5° and Σ ≤ 339.0°, respectively, and the intermediate entries are the cases with Σ in the range 339.0° – 352.5° [23].
| Empirical formula | C21H21ClN3O2P |
|---|---|
| Formula weight | 413.8 |
| Temperature (K) | 94.9(3) |
| Wavelength (Å) | 1.54184 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a (Å) | 10.3249(2) |
| b (Å) | 9.7374(2) |
| c (Å) | 20.3897(4) |
| a (ÃÆââ¬Â¹Ãâà ¡) | 90 |
| b (ÃÆââ¬Â¹Ãâà ¡) | 98.1988(17) |
| g (ÃÆââ¬Â¹Ãâà ¡) | 90 |
| V (Å3) | 2028.98(7) |
| Z | 4 |
| Dcalc (g/cm3) | 1.3548 |
| Absorption coefficient (mm-1) | 2.592 |
| F (000) | 864 |
| Crystal size (mm) | 0.15 × 0.105 × 0.063 |
| q Range for data collection (ÃÆââ¬Â¹Ãâà ¡) | 4.33 to 75.22 |
| Index ranges | –12 ≤ h ≤ 12 |
| –12 ≤ k ≤ 12 | |
| –18 ≤ l ≤ 25 | |
| Reflections collected | 18305 |
| Independent reflections | 4133 [Rint = 0.022] |
| Absorption correction | Multi-scan |
| Max and min transmission | 1.000 and 0.918 |
| Refinement method | full-matrix least-squares on F2 |
| Data/restraints/parameters | 4133/2/262 |
| Goodness-of-fit on F2 | 1.76 |
| Final R indices [I > 3σ(I)] | R1=0.0302, wR2=0.0912 |
| R indices (all data) | R1=0.0327, wR2=0.0933 |
| Largest difference in peak and hole (e Å-3) | 0.34 and –0.28 |
Table 1: Crystal data and structure refinement for [2-Cl-C6H4C(O)NH] P(O)[NHC6H4-4-CH3]2.
| P1-O1 | 1.4770(9) | P1-N1 | 1.6862(11) |
| P1-N2 | 1.6335(11) | P1-N3 | 1.6465(11) |
| C1-O2 | 1.2254(15) | ||
| O1-P1-N1 | 104.42(5) | O1-P1-N2 | 114.04(5) |
| O1-P1-N3 | 118.98(6) | N1-P1-N2 | 113.23(6) |
| N1-P1-N3 | 106.76(5) | N2-P1-N3 | 99.54(5) |
| P1-N1-C1 | 125.72(8) | P1-N2-C9 | 126.74(9) |
| P1-N3-C5 | 127.95(9) |
Table 2: Selected bond distances (Å) and angles (°).
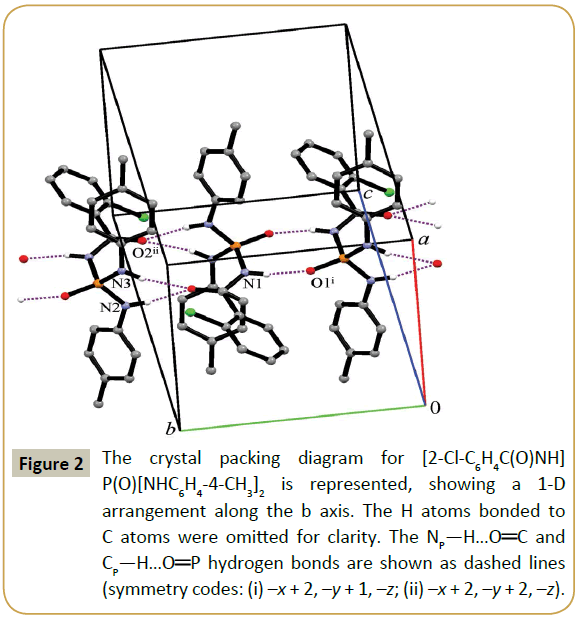
The nitrogen atoms in the title structure do not take part in hydrogen bonding as an acceptor, because they have low Lewisbase character. So, two H-acceptors and three H-donors existing in the structure make three different hydrogen-bonded ring motifs. The more acidic NH of the C(O)NHP(O) moiety (NCPH) participates in an R22(8) motif together with P(O), whereas the two other H atoms of the NHR units (NP) participate in an R22(12) motif combined with R12(6) together with C(O). On the other hand, adjacent molecules are linked via a sequence of alternating R22(8) and R22(12)/R12(6) ring motifs with together C11(4) chain motif in a linear arrangement parallel to b axis (Figure 2). It was found that the strongest NCP—H…O hydrogen bonds exist in the R22(8) motif (Table 3), as in recently published papers [24-33]. In addition to the hydrogen bonds noted, there are also the weak C—H…O interactions (between the phosphoryl oxygen with the neighboring aromatic CÃÆâÃâââ¬Ãâââ¬Â¢H donor of the 2-Cl-C6H4 part) through discrete D11(2) hydrogen bond motifs along the a axis. Further stabilization of this compound is achieved via chlorine atom participating in the C—H…Cl hydrogen bond along the c axis (through D11(2) graph-set motifs). These interactions complete a 3D structure of the title compound.
Figure 2: The crystal packing diagram for [2-Cl-C6H4C(O)NH] P(O)[NHC6H4-4-CH3]2 is represented, showing a 1-D arrangement along the b axis. The H atoms bonded to C atoms were omitted for clarity. The NP—H…OÃÆâÃâââ¬Â¢ÃâÃÂC and CP—H…OÃÆâÃâââ¬Â¢ÃâÃÂP hydrogen bonds are shown as dashed lines (symmetry codes: (i) –x + 2, –y + 1, –z; (ii) –x + 2, –y + 2, –z).
| D—H…A | d(D—H) | d(H…A) | d(D…A) | ÐDHA |
|---|---|---|---|---|
| N1-H1n1…O1i | 0.874(13) | 1.963(13) | 2.8172(13) | 165.6(15) |
| N2-H1n2…O2ii | 0.874(12) | 2.005(12) | 2.8552(14) | 164.1(16) |
| N3-H1n3…O2ii | 0.874(14) | 2.280(15) | 3.0431(14) | 145.8(15) |
| C12-H1c12…O1iii | 0.960 | 2.5388 | 3.319(2) | 138.49 |
| C14-H1c14…Cl1iv | 0.960 | 2.7843 | 3.353(2) | 118.71 |
Table 3: Hydrogen bonds geometries for [2-Cl-C6H4C(O)NH]P(O)[NHC6H4- 4-CH3]2 (Å and °). Symmetry codes: (i) –x + 2, –y + 1, –z (ii) –x + 2, –y + 2, –z iii) x – 1, y, z (iv) –x + 2, y – 1/2, –z + 1/2.
Conclusion
In summary, we reported a new phosphoric triamide, namely, [2-Cl-C6H4C(O)NH]P(O)[NHC6H4-4-CH3]2 that was prepared in good yield and purity by treating 2-chlorobenzoyl phosphoramidic dichloride with p-toluidine. It crystallizes in the monoclinic system with P21/c space group. Adjacent molecules are linked through (NP—H…)2OÃÆâÃâââ¬Â¢ÃâÃÂC and (NCP—H…) (CÃÆâÃâââ¬Ãâââ¬Â¢H…) OÃÆâÃâââ¬Â¢ÃâÃÂP hydrogen bonds in two-dimensional slabs parallel to ab plane. The slabs consist of chains of alternating R22 (8) and R22 (12) / R22 (6) ring motifs (constructed from NÃÆâÃâââ¬Ãâââ¬Â¢H…O hydrogen bonds) along b axis, which are connected with discrete D11 (2) motifs (of CÃÆâÃâââ¬Ãâââ¬Â¢H…O) along a axis. Weak C—H…Cl hydrogen bonds connect the slabs in the c axis direction, giving rise to a three – dimensional supramolecular structure.
Acknowledgements
Financial support of this work by Ferdowsi University of Mashhad is gratefully acknowledged (Project No. 28383/3). The X-ray part of the work was carried out with the support of Czech Science Foundation, grant GACR 15-12719S using instruments of the ASTRA lab established within the Operation program Prague Competitiveness - project CZ.2.16/3.1.00/24510.
References
- Bollinger JC, Levy-Serpier J, Debord J, Penicaut B (1990) Acetylcholinesterase inhibition by two phosphoric 4-nitroanilides. J Enzyme Inhib Med Chem 3: 211-217.
- Andrews RK, Dexter A, Blakeley RL, Zerner B (1986) Jack Bean Urease (EC 3.5.1.5).8.On the inhibition of urease by amides and esters of phosphoric acid. J Am Chem Soc 108: 7124-7125.
- Gholivand K, Ebrahimi Valmoozi AA, Mahzouni HR, Ghadimi S, Rahimi R, et al. (2013) Molecular docking and QSAR studies: noncovalent interaction between acephate analogous and the receptor site of human acetylcholinesterase. J Agric Food Chem 61: 6776-6785.
- Fu Z, Chivers T (2007) Solvent effects on the reactions of copper chlorides with OP(NH-t-Bu)3 – Formation of the novel [Cu5Cl10]5– anion via in situ templation. Can J Chem 85: 358-365.
- Pourayoubi M, Golen JA, Rostami Chaijan M, Divjakovic V, Negari M, et al. (2011) The hydrogen-bonded dimers of N,NÃÆà  Ãâù,NÃÆà  Ãâú-tricyclohexylphosphoric triamide in new tin(IV) and copper(II) complexes. Acta Crystallogr C67: 160-164.
- Gholivand K, Mostaanzadeh H, Koval T, Dušek M, Erben MF, et al. (2010) Syntheses, spectroscopic study and X-ray crystallography of some new phosphoramidates and lanthanide(III) complexes of N-(4-nitrobenzoyl)-NÃÆà  Ãâù, NÃÆà  Ãâú-bis(morpholino)phosphoric triamide. Acta Crystallogr B 66: 441-450.
- Gholivand K, Mahzouni HR, Pourayoubi M, Amiri S (2010) High-coordinated lanthanum(III) complexes with new mono- and bidentate phosphoryl donors; spectroscopic and structural aspects. Inorg Chim Acta 363: 2318-2324.
- Trush EA, Amirkhanov VM, Ochynnikov VA, Swiatek-Kozlowska J, Lanikina KA, et al. (2003) Metal carbacylamidophosphates: ability of coordination patterns to di- and polymerization. Polyhedron 22: 1221-1229.
- Gholivand K, Pourayoubi M, Shariatinia Z (2007) coupling constants dependency on the ring size, hybridization, and substituents in new diazaphospholes and diazaphosphorinanes, NMR and X-ray crystallography studies. Polyhedron 26: 837-844.
- Pourayoubi M, Sabbaghi F (2009) Synthesis, spectroscopic characterization and crystal structure of a new acetyl phosphorylamidate P(O)[NHC(O)C6H4(4-NO2)][N(CH(CH3)2)(CH2C6H5)]2. J Chem Crystallogr 39: 874–880.
- Pourayoubi M, Shoghpour S, Bruno G, Amiri Rudbari H (2011) N-(3-Fluorobenzoyl)-NÃÆà  Ãâù,NÃÆà  Ãâú-bis(4-methylphenyl)phosphoric triamide. Acta Crystallogr E67: 3034.
- Pourayoubi M, Shoghpour S, Bruno G, Amiri Rudbari H (2011) N,NÃÆà  Ãâù-Dicyclohexyl-NÃÆà  Ãâú-(3-fluorobenzoyl)-N, NÃÆà  Ãâù-dimethylphosphoric triamide. Acta Crystallogr E67: 3028-3029.
- Rigaku Oxford Diffraction (2015) CrysAlisPRO, Rigaku Corporation, Tokyo, Japan.
- Palatinus L, Chapuis G (2007) SUPERFLIP – a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J Appl Crystallogr 40: 786–790.
- PetÃÆââ¬Â¦Ãâââ¢íÃÆââ¬Å¾ÃâÃÂek V, Dušek M, Palatinus L (2014) Crystallographic computing system JANA2006: general features. Z Kristallogr 229: 345-352.
- Kirsanov AV, Makitra RG (1956) Dichlorides of acylamidophosphoric acids of aromatic series. Zh Obshch Khim 26: 905-907.
- Rudd MD, Lindeman SV, Husebye S (1996) Structural characteristics of three-coordinate arylhalide tellurium(II) complexes with chalcogen ligands. Synthesis, spectroscopic characterization and X-ray structural studies of bromo[N-methylbenzothiazole-2(3H)selone]phenyltellurium(II),bromophenyl[tris(dimethylamino)phosphaneselenide]tellurium(II) and tris(dimethylamino)phosphanesulfide. Acta Chem Scand 50: 759-774.
- Corbridge DEC (1995) Phosphorus: An outline of its chemistry, biochemistry and technology. 5th edn. Amsterdam: Elsevier Science.
- Gilheany DG (1994) No d orbitals but Walsh diagrams and maybe banana bonds: chemical bonding in phosphines, phosphine oxides, and phosphonium Ylides. Chem Rev 94: 1339-1374.
- Pourayoubi M, Jasinski JP, Shoghpour Bayraq S, Eshghi H, Keeley AC, et al. (2012) Three new [XC(O)NH]P(O)[N(CH2-C6H5)2]2 phosphoric triamides (X=CClF2, 3-F-C6H4 and 3,5-F2-C6H3): a database analysis of tertiary N-atom geometry in compounds with a C(O)NHP(O)[N]2 core. Acta Crystallogr C68: 399-404.
- Pourayoubi M, Toghraee M, Divjakovic V, van der Lee A, Mancilla Percino T, et al. (2013) Analysis of N—H…O hydrogen bonds in new C(O)—NH—P(O)-based phosphoric triamides and analogous structures deposited in the Cambridge Structural Database. Acta Crystallogr B69: 184-194.
- Pourayoubi M, NeÃÆââ¬Å¾ÃâÃÂas M, Negari M (2012) The double H-atom acceptability of the PÃÆâÃâââ¬Â¢ÃâÃÂO group in new XP(O)(NHCH2C6H4-2-Cl)2 phosphoramidates [X = C6H5O– and CF3C(O)NH–]: a database analysis of compounds having a P(O)(NHR) group. Acta Crystallogr C68: 51-56.
- Allen FH, Bruno IJ (2010) Bond lengths in organic and metal-organic compounds revisited: X—H bond lengths from neutron diffraction data. Acta Crystallogr B66: 380-386.
- Pourayoubi M, Tarahhomi A, Rheingold AL, Golen JA (2010) N,N'-Dibenzyl-N''-(2,6-difluorobenzoyl)-N,N'-dimethylphosphoric triamide. Acta Crystallogr E66: 2524.
- Pourayoubi M, Tarahhomi A, Rheingold AL, Golen JA (2010) N,NÃÆà  Ãâù-Di-tert-butyl-NÃÆà  Ãâú-(2,6-difluorobenzoyl)phosphoric triamide. Acta Crystallogr E66: 3159.
- Pourayoubi M, Tarahhomi A, Rheingold AL, Golen JA (2011) N-(2-Fluorobenzoyl)-NÃÆà  Ãâù,NÃÆà  Ãâú-bis(4-methylphenyl)phosphoric triamide. Acta Crystallogr E67: 934.
- Pourayoubi M, Rostami Chaijan M, Torre-Fernández L, García-Granda S (2011) N-Benzoyl-NÃÆà  Ãâù,NÃÆà  Ãâú-dicyclohexylphosphoric triamide. Acta Crystallogr E67: 1360.
- Pourayoubi M, Rostami Chaijan M, Torre-Fernández L, García-Granda S (2011) N,NÃÆà  Ãâù-Dibenzyl-N,NÃÆà  Ãâù-dimethyl-NÃÆà  Ãâú-(4- nitrobenzoyl)phosphoric triamide. Acta Crystallogr E67: 1031.
- Pourayoubi M, Saneei A (2011) N-(2-Chloro-2,2-difluoroacetyl)-NÃÆà  Ãâù,NÃÆà  Ãâú- diisopropylphosphoric triamide. Acta Crystallogr E67: 665.
- Pourayoubi M, Toghraee M, Divjakovic V (2011) N,NÃÆà  Ãâù-Bis(2-chlorobenzyl)-NÃÆà  Ãâú-(dichloroacetyl)phosphoric triamide. Acta Crystallogr E67: 333.
- Raissi Shabari A, Pourayoubi M, Saneei A (2011) N,NÃÆà  Ãâù-Dibenzyl-NÃÆà  Ãâú-(2-chloro-2,2-difluoroacetyl)-N,NÃÆà  Ãâù-dimethylphosphoric triamide. Acta Crystallogr E67: 663-664.
- Tarahhomi A, Pourayoubi M, Rheingold AL, Golen JA (2011) Different orientations of C=O versus P=O in P(O)NHC(O) skeleton: the first study on an aliphatic diazaphosphorinane with a gauche orientation. Struct Chem 22: 201-210.
- Toghraee M, Pourayoubi M, Divjakovic V (2011) Study on H-bond patterns in phosphoric triamides having a P(O)NHC(O) skeleton, a gauche orientation of P(O) vs C(O) in new compounds. Polyhedron 30: 1680-1690.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences