Synthesis, Characterization and Crystal Structure of 1,4,5,8-Tetrakis(Perfluoropyridin4-yloxy) Naphthalene
Khalil Beyki, Werner Kaminsky, Reza Heydari and Malek Taher Maghsoodlou
DOI10.21767/2470-9905.100027
Khalil Beyki1*, Werner Kaminsky2, Reza Heydari1 and Malek Taher Maghsoodlou1
1Department of Chemistry, Faculty of Science, University of Sistan and Baluchestan, PO Box 98135-674, Zahedan, Iran
2Department of Chemistry, Departmental of Crystallographer, University of Washington, PO Box 351700, Seattle, USA
- *Corresponding Author:
- Khalil Beyki
Department of Chemistry, Faculty of Science, University of Sistan and Baluchestan
PO Box 98135-674, Zahedan
Iran
Tel: +61449205856
Email:chemkhalil@pgs.usb.ac.ir
Received date: April 10, 2017; Accepted date: April 12, 2017; Published date: April 17, 2017
Citation: Beyki K, Kaminsky W, Heydari R, et al. Synthesis, Characterization and Crystal Structure of 1,4,5,8-Tetrakis(Perfluoropyridin- 4-yloxy) Naphthalene. Struct Chem Crystallogr Commun. 2017, 3:1.
Abstract
1,4,5,8-tetrakis(perfluoropyridin-4-yloxy)naphthalene (tetra-PPN) was synthesized via a one-pot pseudo-five-component reaction of pentafluoropyridine and 5,8-dihydroxy-1,4-tetralindione under basic conditions in DMF. The asymmetric unit contains one half-mol ecule of tetra-PPN and data reveals that half of the molecule being related to the other via inversion symmetry. A detailed analysis of the intermolecular interactions of the compound has been performed using Hirshfeld surfaces. The optimized geometrical parameters and calculated spectroscopic features obtained by quantum chemical calculations using density functional (DFT) methods. In addition, to support the experimental results, the 19F NMR values are obtained using B3LYP method. The obtained results are compared with experimental values show a very good agreement with the experimental data.
Keywords
Pentafluoropyridine; Tetralindione; One-pot synthesis; Single crystal; X-ray structure; DFT
Introduction
Organofluorine compounds find many different applications, ranging from pharmaceuticals and agrochemicals to advanced materials and polymers [1]. The unique properties of the ÃÆïÃâìÃâââ¬Å¡uorine atom, such as high electronegativity, low polarizability, and small covalent radius together with the great strength of the C-F bond and its tendency to increase lipophilicity of organic molecules are behind its wide impact. Circa 30−40% of agrochemicals and 20% of pharmaceuticals contain at least one ÃÆïÃâìÃâââ¬Å¡uorine atom [2,3].
Many perfluoroheteroaromatic compounds such as perÃÆïÃâìÃâââ¬Å¡uoroquinoline [4] and pentafluoropyridine, in which all the hydrogen atoms of the heterocyclic rings have been replaced by fluorine atoms, have been investigated since the early 1960s [2,5,6]. The most important reaction of pentaÃÆïÃâìÃâââ¬Å¡uoropyridines involves the replacement of the para-ÃÆïÃâìÃâââ¬Å¡uorine atom by nucleophilic reagents allowing the synthesis of new organofluorine compounds such as heterocyclic and macrocyclic perfluoro systems [7-9]. Theoretical calculations were done using the density functional theory (DFT) with B3LYP methods using 6-31+G (d,p) basis set to support our spectroscopic and structural properties of the studied molecule. The geometric structural parameters and 19F NMR results were calculated and compared with the experimental results.
Results and Discussion
Previously we have described the synthesis of some 4-substituted tetrafluoropyridine systems from reaction of pentaÃÆïÃâìÃâââ¬Å¡uoropyridine with tetrazole-5-thiol, piperazine, hydroxylated naphtoquinones and antraquinones [10-12]. Here we describe the reaction of pentafluoropyridine with 5,8-dihydroxy-1,4-tetralindione, resulting in 1,4,5,8-tetrakis(perfluoropyridin-4-yloxy)naphthalene (tetra-PPN) (Figure 1). Whilst many reactions of nucleophiles with pentafluoropyridine have been reported, processes involving one-pot pseudo five-component reactions of pentafluoropyridine with nucleophiles are uncommon.
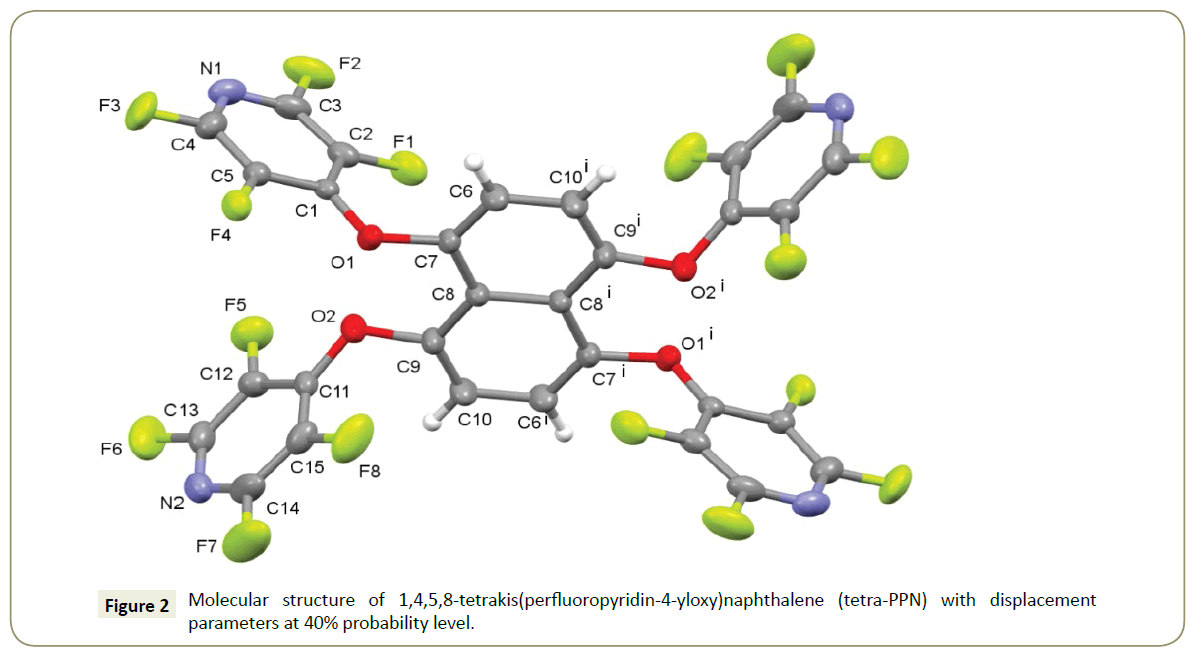
When 5,8-dihydroxy-1,4-tetralindione was treated with pentafluoropyridine in the presence of potassium carbonate in DMF tetra-PPN was formed in moderate yield. The singlecrystal X-ray structure of the structure (tetra-PFN) (1) is shown in Figure 2. Structure crystallizes in the monoclinic crystal system with space group P21/n which appears to be centrosymmetric, with one half of the molecule being related to the other via inversion symmetry. Further details of the structure determination are given in Table 1.
| Empirical formula | C30 H4 F16 N4 O4 |
|---|---|
| Formula weight | 788.37 |
| Temperature | 292(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P 21/n |
| Unit cell dimensions | a = 5.7900(2) Å |
| b = 18.8129(8) Å | |
| c = 13.3418(5) Å | |
| Volume | 1435.19(10) Å3 |
| Z | 2 |
| Density (calculated) | 1.824 Mg/m3 |
| Absorption coefficient | 0.192 mm-1 |
| F(000) | 776 |
| Crystal size | 0.50 x 0.10 x 0.07 mm3 |
| Theta range for data collection | 2.17 to 28.26°. |
| Index ranges | -7<=h<=7, -24<=k<=24, -17<=l<=17 |
| Reflections collected | 6319 |
| Independent reflections | 3427 [R(int) = 0.0741] |
| Completeness to theta = 25.00° | 99.0 % |
| Max. and min. transmission | 0.9867 and 0.9100 |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 3427 / 0 / 245 |
| Goodness-of-fit on F2 | 0.974 |
| Final R indices [I>2sigma(I)] | R1 = 0.0687, wR2 = 0.1717 |
| R indices (all data) | R1 = 0.1856, wR2 = 0.2229 |
| Extinction coefficient | 0.018(5) |
| Largest diff. peak and hole | 0.335 and -0.215 e.Å-3 |
Table 1: Crystallographic data for the structures provided.
The average distance between neighbouring fluorine atoms is 2.90 Å length. An intermolecular quasi-hydrogen bond C10- HÃÆâÃâÃâ Ãâââ¢ÃÆâÃâÃâ Ãâââ¢ÃÆâÃâÃâ Ãâââ¢N1 connects (Hydrogen-Acceptor distance=2.76(1) Å) the pyridine unit with the naphthalene. However, the second pyridine nitrogen N2 exhibits a σ-π interaction with a neighbour pyridine. The molecular packing is given in Figure 3.
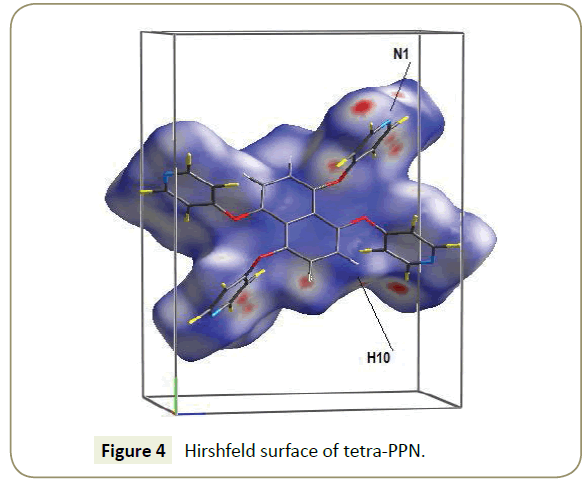
The intermolecular interactions are illustrated by a Hirschfeld surface [13-15], which is constructed from points with fixed ratio (usually 0.5) between the contribution to the electron density from a molecule and the contribution from electron density from the whole crystal. Particularly instructive is to colour-code the surface by the distance to the nearest atom within the surface or external to it. Short interatomic distances on both sides on the surface are plotted red, longer ones in blue (Figure 4). Van der Waals interactions are marked red (N1, H10). No red marking on the surface was found in the vicinity of N2.
In the calculation, the initial geometry of the molecule was obtained from X-ray results and it was optimized using B3LYP method with B3LYP basis set. The optimized structure is given in Figure 5. The selected bond lengths, bond angles are given in Table 2. As seen in Table 1, and chart 1 and 2, the obtained values are in good agreement with the experimental values. The maximum deviation is 0.019 Å for C12-F4 bond length and 3.62o for F8-C15-C11 bond angle. In addition, the orientations of the tetrafluoronaphthalene rings on the planar naphthalene ring are 27.04o (C9-O2-C11-C15) and 47.53o (C7-O1-C1-C2).
| Bond lengths | Bond angles | ||||
|---|---|---|---|---|---|
| Exp. | Calc. | Exp. | Calc. | ||
| C9-O2 | 1.403(4) | 1.397 | C9-O2-C11 | 118.6(2) | 121.9 |
| O2-C11 | 1.348(4) | 1.351 | F8-C15-C11 | 117.6(2) | 121.2 |
| C15-F8 | 1.345(5) | 1.345 | F5-C11-C12 | 122.0(2) | 119.8 |
| C14-F7 | 1.331(6) | 1.338 | F6-C13-N2 | 115.2(2) | 117.4 |
| C13-F6 | 1.335(5) | 1.337 | N2-C14-F7 | 119.3(2) | 117.5 |
| C12-F5 | 1.322(4) | 1.341 | C7-O1-C1 | 120.0(3) | 120.7 |
| O1-C7 | 1.408(4) | 1.392 | O1-C1-C2 | 127.3(3) | 124.2 |
| C1-O1 | 1.354(4) | 1.355 | C1-C2-F1 | 121.2(4) | 120.5 |
| C2-F1 | 1.344(4) | 1.342 | C2-C3-F2 | 117.9(3) | 118.5 |
| C3-F2 | 1.347(5) | 1.337 | F2-C3-N1 | 116.0(3) | 117.5 |
| C4-F3 | 1.353(5) | 1.337 | N1-C4-F3 | 117.0(2) | 117.5 |
| C5-F4 | 1.342(4) | 1.340 | F3-C4-C3 | 118.3(2) | 118.7 |
| C4-C5-F4 | 121.3(2) | 121.4 | |||
| Dihedrals | |||||
| C9-O2-C11-C15 | 60.7(2) | 27.04 | |||
| C7-O1-C1-C5 | -160.3(3) | -137.53 | |||
Table 2: Selected bond lengths (Å) and angles (°) with the selected torsion angles (°) for tetra-PPN. The calculated values are for gas phase.
NMR spectroscopic characterization
Tetra-PPN was characterized by 19F NMR data. It is obtained that the experimantal and calculated values are compatible with each other. The fluorine atoms located in the ortho position relative to the ring nitrogen has a chemical shift of -87.61 ppm (calc. -106.66 ppm) and -91.69 ppm (calc. -110.16 ppm) and the fluorine resonance located in the meta position occur at -156.69 ppm (calc. -173.94 ppm) and -162.54 ppm ppm (calc. -179.69 ppm). In Tetra-PPN, two chemical shift of ortho and meta position, there may be hindered rotation about the C-O bonds. The 1H NMR spectra of tetra-PFN shows the protons of the naphthyl ring at δ=7.25 ppm.
X-ray crystallography
The single-crystal X-ray crystallographic diffraction data were collected at 293 K with a Rigaku R-axis Rapid-S IP-detector diffractometer with graphite-monochromated Mo-Kα radiation (λ=0.71073 Å). Suitable single crystal of tetra-PPN was mounted on the tip of a glass fiber with silicone grease and transferred to the diffractometer for data collection. The collection of frames of data, indexing of reflections, and determination of the lattice parameters and the integration of the intensity of the reflections were performed with the CrystalClear (Rigaku/MSC Inc., 2005) software [16]. All of the structures were solved by direct methods with SHELXS-97 [17,18] and refined with SHELXL-97 [19,20]. The non-hydrogen atoms were refined anisotropically, and the hydrogen atoms were fixed at calculated positions and refined isotropically with a riding model. The final difference Fourier maps showed no peaks of chemical significance. The crystallographic data are summarized in Table 2. CCDC 1521820 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
Computational methods
The calculations were performed using the Gaussian 09 package [21]. The geometries of the molecule has been fully optimized in the ground state at the B3LYP/6-31+G(d,p) computational level [22-24]. The vibrational frequencies were calculated at the same theoretical level to confirm that all optimized ground configurations had no imaginary frequencies and the groundstate minima were on potential energy surfaces. The 19F NMR chemical shifts were obtained using the Gauge-Including Atomic Orbital (GIAO) method [25,26] with B3LYP/6-311++G(d, p) method in CDCl3 solvent. The values of the chemical shifts were estimated using δCCl3F value for shielding constant of trichlorofluoromethane (CCl3F) florine obtained by the same approach, according to expression δcald.=δCCl3F-δ.
Conclusion
We have demonstrated that all four oxygen atoms in tetralindione can react with pentafluoropyridine to afford of 1,4,5,8-tetrakis(perfluoropyridin-4-yloxy)naphthalene, which was characterized spectroscopically and via single crystal X-ray diffraction, in a one-pot synthesis.
Experimental
General information
All materials and solvents, purchased from Merck and Aldrich, were used without any additional purification. The melting points of the products were determined in open capillary tubes using BAMSTEAB Electrothermal apparatus model 9002. The 1H NMR spectra were recorded at 300 MHz and referenced to TMS. The 19F-NMR spectra were recorded at 376 MHz. In the 19F-NMR spectra, up field shifts were quoted as negative and referenced to CFCl3. Silica plates (Merck) were used for TLC analysis.
Preparation of 1,4,5,8-tetrakis(perfluoropyridin- 4-yloxy)naphthalene (tetra-PPN)
Pentafluoropyridine (0.32 g, 2 mmol), 5,8-dihydroxy-1,4- tetralindione (0.1 g, 0.5 mmol) and potassium carbonate (0.5 g, 4.0 mmol) were stirred in DMF (5 mL) at reflux temperature for 6 h. The reaction mixture was evaporated to dryness and the solid product was then recrystallized from ethyl acetate and chloroform to give tetra-PPN (0.3 g, 45%) as red crystals; mp 380°C dec.
1H NMR (CDCl3): δ (ppm) 7.25 (s, 4H, CH) 19F NMR (CDCl3) δ (ppm) -87.61 (s, 4F, F-2,6), -91.69 (s, 4F, F-2',6'), -156.69 (s, 4F, F-3,5), -162.54 (m, 4F, F-3',5').
Conflict of Interest
None declared under financial, general, and institutional competing interests. I wish to disclose a competing interest(s) such as those defined above or others that may be perceived to influence the results and discussion reported in this paper.
References
- Hunter L (2010) The C-F bond as a conformational tool in organic and biological chemistry. Beilstein Journal of Organic Chemistry 6: 38.
- Champagne PA, Desroches J, Hamel JD, Vandamme M, Paquin JF,et al. (2015) Monofluorination of organic compounds: 10 years of innovation. Chemical Reviews 115: 9073-9174.
- Yin X, Guo Y, Liu C, Wang Z, Zhang B, et al. (2015) One-pot two step facile synthesis of 2, 3, 5, 6-tetrafluorobenzonitrile-containing dithiocarbamic acid esters. Tetrahedron Letters 56: 5135-5139.
- Fox MA, Pattison G, Sandford G, Batsanov AS (2013) 19 F and 13 C GIAO-NMR chemical shifts for the identification of perfluoroquinoline and-isoquinoline derivatives. Journal of Fluorine Chemistry 155: 62-71.
- Cartwright MW, Parks EL, Pattison G, Slater R, Sandford G, et al. (2010) Annelation of perfluorinated heteroaromatic systems by 1,3-dicarbonyl derivatives. Tetrahedron 66: 3222-3227.
- Christopher JA, Brophy L, Lynn SM, Miller DD, Sloan LA, et al. (2008) Synthetic utility of 4-bromo-2, 3, 5, 6-tetrafluoropyridine. Journal of Fluorine Chemistry 129: 447-454.
- Ojima I (2009) Fluorine in medicinal chemistry and chemical biology. John Wiley & Sons, pp: 71-639.
- Ranjbar-Karimi R, Mashak-Shoshtari M, Hashemi-Uderji S, Kia R (2011) Synthesis and structural study of bis-perfluoropyridyl bridged by 1, 4 and 1, 2 dihydropyridine. Journal of Fluorine Chemistry 132: 285-290.
- Chambers RD, Khalil A, Murray CB, Sandford G, Batsanov AS, et al. (2005) Polyhalogenated heterocyclic compounds: Part 52. [1] Macrocycles from 3, 5-dichloro-2, 4, 6-trifluoropyridine. Journal of Fluorine Chemistry 126: 1002-1008.
- Beyki K, Haydari R, Maghsoodlou MT (2015) Synthesis of 2, 3, 5, 6-tetrafluoro-pyridine derivatives from reaction of pentafluoropyridine with malononitrile, piperazine and tetrazole-5- thiol. SpringerPlus 4: 757.
- Reza RK, Roghayeh D, Khalil B (2015) Arabian Journal of Chemistry.
- Beyki K, Haydari R, Maghsoodlou MT (2016) Reaction of hydroxylated naphtoquinones/antraquinones with pentafluoropyridine. SpringerPlus 5: 110.
- McKinnon JJ, Mitchell AS, Spackman MA (1998) Hirshfeld surfaces: a new tool for visualising and exploring molecular crystals. Chemistry – A European Journal 4: 2136-2141.
- Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. Cryst Eng Comm 4: 378-392.
- McKinnon JJ, Spackman MA, Mitchell AS (2004) Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallographica Section B: Structural Science 60: 627-668.
- Rigaku MSC Inc. (2017) 9009 New Trails Drive, The Woodlands,TX, USA.
- Sheldrick GM (1997) SHELXL-97, Program for the Refinement of Crystal Structures. University of Göttingen, Germany.
- Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallographica Section C: Structural Chemistry 71: 3-8.
- Mackay S, Edwards C, Henderson A, Gilmore C, Stewart N, et al. (1997) Maxus: a computer program for the solution and refinement of crystal structures from diffraction data. University of Glasgow, Scotland.
- Waasmaier D, Kirfel A (1995) New analytical scattering-factor functions for free atoms and ions. Acta Crystallographica Section A: Foundations of Crystallography 51: 416-431.
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, et al. (2009) Gaussian 09. Revision A.2. Wallingford CT: Gaussian Inc.
- Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density.Physical review B 37: 785.
- Becke AD (1993) DensityÃÆâÃâââ¬ÃâÃÂfunctional thermochemistry.III. The role of exact exchange. The Journal of chemical physics 98: 5648-5652.
- Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theoretical Chemistry Accounts: Theory, Computation, and Modelling Theoretica Chimica Acta 28: 213-222.
- Ditchfield R (1974) Self-consistent perturbation theory of diamagnetism: I. A gauge-invariant LCAO method for NMR chemical shifts. Molecular Physics 27: 789-807.
- Wolinski K, Hinton JF, Pulay P (1990) Efficient implementation of the gauge- independent atomic orbital method for NMR chemical shift calculations. Journal of the American Chemical Society 112: 8251-8260.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences