Sarcosinium and Betainium Picrates
Petrosyan AM, Ghazaryan VV, Giester G and Fleck M
Petrosyan AM1*, Ghazaryan VV1, Giester G2 and Fleck M2
1Institute of Applied Problems of Physics, NAS of Armenia, 25 Nersessyan St., 0014 Yerevan, Armenia
2Institute of Mineralogy and Crystallography, University of Vienna, Althanstr. 14, A-1090 Vienna, Austria
- *Corresponding Author:
- Petrosyan AM
Institute of Applied Problems of Physics, NAS of Armenia, 25 Nersessyan St., 0014 Yerevan, Armenia
Tel: +37410241106
E-mail:aram.m.petrosyan@gmail.com, apetros@iapp.sci.am
Received Date: December 28, 2015 Accepted Date: January 04, 2016 Published Date: January 09, 2016
Citation: Petrosyan AM, Ghazaryan VV, Giester G, et al. The Structure of Metals and its Influence on Metallic Properties. Struct Chem Crystallogr Commun. 2016, 2:1.
Abstract
Reactions of sarcosine (N-methylglycine) and betaine (N-trimethylglycine) with picric acid in aqueous media were studied in comparison with previously studied reaction of glycine with picric acid. In contrast to glycine, where glycine glycinium picrate salt with dimeric cation is formed at both 2:1 and 1:1 molar ratios, in cases of sarcosine and betaine reactions at both 2:1 and 1:1 molar ratios lead to formation of sarcosinium picrate and betainium picrate. Crystal and molecular structures of sarcosinium picrate and betainium picrate were determined by single crystal X-ray diffraction analysis at 200 K. Both salts crystallized in monoclinic system (space groups P21/c and C2/c respectively). In both cases asymmetric units contain one formula unit and carboxyl groups of cations form hydrogen bonds with phenolic oxygen atoms of picrate anions with O···O distances 2.5861(13) Å and 2.5265(13) Å respectively. Similarity and dissimilarity of two structures are analyzed and discussed. Vibrational (infrared and Raman) spectra of both salts were recorded and discussed.
Keywords
Sarcosinium picrate; Betainium picrate; Crystal structure; Vibrational spectra
Introduction
Salts of amino acids are interesting in many respects [1]. In addition to simple salts, salts (A···A+)·X– with dimeric cation, where X– is an anion, and A and A+ are amino acids in the zwitterionic and cationic states, respectively, linked by a short O-H···O hydrogen bond, are known for many amino acids. These salts are of interest in themselves as crystals with short hydrogen bonds, but also as crystals with possible nonlinear optical and ferroelectric properties. For the simplest amino acid glycine (Gly) significant number of salts with dimeric cation is known. The methylderivatives of glycine (N-monomethylglycine, sarcosine (Sar) and N-trimethylglycine, betaine (Bet)) are also known as capable forming such salts. Particularly, salts of (A···A+)·X– type for glycine, sarcosine and betaine are known with chloride, bromide, iodide, nitrate, tetrafluoroborate and perchlorate anions [1]. Additionally, the crystal glycine glycinium picrate is also known [2]. In this respect it was interesting to investigate reactions of sarcosine and betaine with picric acid to know what kind of salts is formed in these cases.
Experimental
Synthesis
As initial reagents sarcosine (crystallized, ≥ 98% (T)) and betaine (in form of monohydrate, ≥ 99% (NT)) purchased from Sigma- Aldrich Chem. Co. and picric acid “pure” (“KWAS PIKRYNOWY” cz. Zaklady Chemiczne, Krupski Mlyn, Poland) were used without further purification. We supposed formation of salts of (A···A+)·X– type with dimeric cation and/or A+·X– type simple salts. Salts were obtained by slow evaporation of aqueous solutions with definite amino acid: acid ratios at room temperature. Salts obtained from solutions with 2.5:1, 2:1, 1:1 ratios of sarcosine (betaine) and picric acid were identical in each case based on their infrared spectra. Structure determination showed that in both cases simple salts of A+·X– type, namely, sarcosinium picrate and betainium picrate are formed. This is in contrast to the case of the reaction of glycine with picric acid when at 2:1, 1:1 ratios the salt of (A···A+)·X– type, namely, glycine glycinium picrate is formed.
Structure determination
Suitable single crystals of the title compounds were mounted on glass needles with laboratory grease and measured on a Bruker APEXII CCD diffractometer equipped with an Incoatec Microfocus Source IμS (30 W, multilayer mirror, Mo-Kα) at 200 K (LT device Cryostream 800; Oxford Cryosystems). The data were processed using the diffractometer Software package, the structure was solved using Direct methods employing the program SHELXS97 [3-5]. Subsequent difference Fourier synthesis and leastsquare refinement were used to determine the positions of the remaining non-hydrogen atoms. These atoms were refined with independent anisotropic displacement parameters. Hydrogen atoms were placed in geometrically calculated positions and their displacement parameters were fixed to be 1.5 times larger than those of the parent atom. The successful convergence of the refinement was shown by the maximum shift/error of 0.001 for the last cycle of the least-squares refinement. Data collection and structure refinement parameters and crystallographic data for (I) and (II) are listed in Table 1. Further crystallographic data have been deposited with the Cambridge Crystallographic Data Centre and can be obtained free of charge via www.ccdc.cam.ac.uk/ conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, Fax: +441223336033), citing the title of this paper and the CCDC nos. 1443913 and 1443914.
| Formula | (I) | (II) |
|---|---|---|
| C9H10N4O9 | C11H14N4O9 | |
| Mr | 318.21 | 346.26 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | C2/c |
| a (Å) | 7.1067(14) | 19.863(4) |
| b (Å) | 8.3007(17) | 6.6894(13) |
| c (Å) | 22.021(4) | 24.216(5) |
| a (°) | 90.00 | 90.00 |
| b (°) | 96.53(3) | 113.86(3) |
| g (°) | 90.00 | 90.00 |
| V (Å3), Z | 1290.6(4), 4 | 2942.7(10), 8 |
| Dcalc (gcm-3) | 1.638 | 1.563 |
| m (Mo Kα) (cm-1) | 0.149 | 0.138 |
| F (000) | 656 | 1440 |
| T (K) | 200(2) | 200(2) |
| hkl range | ± 10, ± 12, ± 33 | ± 30, ± 10, ± 37 |
| Reflections measured | 35339 | 46005 |
| Reflections unique | 4908 | 5618 |
| Data with (Fo>2s (Fo)) | 3946 | 4187 |
| Rint | 0.0282 | 0.0442 |
| Parameters refined | 229 | 227 |
| R(F)* (forFo>2s (Fo)) | 0.0418 | 0.0486 |
| wR(F2)* (all reflections) | 0.1225 | 0.1315 |
| Weighting parameters a, b | 0.060/0.363 | 0.057/2.39 |
| Δρfin (max/min) [e Å–3] | 0.451/-0.417 | 0.797/-0.406 |
Table 1 Crystal data and details of the refinement for sarcosinium picrate (I) and betainium picrate (II).
Vibrational spectra
Attenuated total reflection Fourier-transform infrared spectra (FTIR ATR) were recorded by a Nicolet 5700 spectrometer (ZnSe prizm, Happ-Genzel apodization, ATR distortion is corrected, number of scans 32, resolution 4 cm-1). Parts of the IR spectra in the region 500–400 cm-1 were taken from FTIR spectra recorded with Nujol mull (4000–400 cm-1, number of scans 32, resolution 4 cm-1. Fourier-transform Raman spectra were recorded by a NXR FT-Raman Module of a Nicolet 5700 spectrometer at room temperature with resolution 4 cm-1. Number of scans and laser power at the sample were 128 and 0.21 W for (I) and (II).
Results and Discussion
Crystal and molecular structures of sarcosinium picrate and betainium picrate
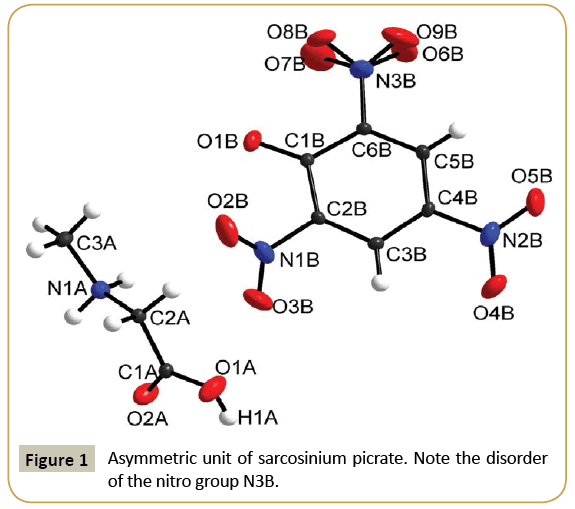
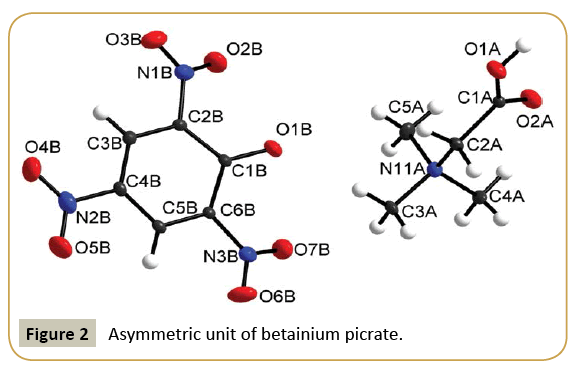
Sarcosinium picrate (I) and betainium picrate (II) crystallize in monoclinic system with P21/c and C2/c space groups, respectively. Crystallographic data and details of structure refinements are provided in Table 1. Asymmetric units in each case contain sarcosinium (betainium) cation and picrate anion (Figures 1 and 2). One of nitro group of picrate anion in the structure of (I) is disordered (Figure 1). Intramolecular bond distances and angles of both crystals are provided in Table 2. Parameters of hydrogen bonds are presented in Table 3 and packing diagrams in Figures 3 and 4. Bond lengths and angles of carboxyl groups have expected values. In both structures carboxyl groups of cations form hydrogen bond with phenolic oxygen atoms of picrate anions. The main distinction in hydrogen bonds is the presence of additional N-H···O hydrogen bonds of NH2 + group of sarcosinium cation. Hydrogen bond O1A-H1A···O1B formed by betainium cation is stronger (O···O distance makes 2.5265(13) Å) than respective hydrogen bond formed by sarcosinium cation (O···O distance makes 2.5861(13) Å) (Table 3). The values of C-OH bond lengths (1.3029(12) Å in (I) and 1.3134(15) Å in (II) (Table 2)) are not in accordance with correlation between C-OH bond lengths vs. O···O distances [6], while the values of O2A-C1A-O1A, O2-C1-C2A and O1A-C1A-C2A angles are in good agreement with correlation of these angles vs O···O distances [6]. The NH2 + group of sarcosinium cation forms the hydrogen bond N1A-H31···O1B with phenolic oxygen atom of picrate anion and the hydrogen bond N1A-H32···O2A with carbonyl oxygen atom of nearest symmetryrelated sarcosinium cation (Table 3). Thus, phenolic oxygen atom of picrate anion in the structure of (I) forms two hydrogen bonds, while in case of (II) it forms only one hydrogen bond. This may be the cause of longer C1B-O1B distance (1.2660(11) Å) in case of (I) compared with respective distance (1.2541(14) Å) in case of (II).
| (I) | (II) | |
|---|---|---|
| C1A-O1A | 1.3029(12) | 1.3134(15) |
| C1A-O2A | 1.2037(12) | 1.2065(14) |
| C1A-C2A | 1.5089(14) | 1.5222(16) |
| C2A-N1A | 1.4798(12) | 1.4960(15) |
| N1A-C3A | 1.4826(13) | 1.5046(16) |
| N1A-C4A | - | 1.4987(15) |
| N1A-C5A | - | 1.4939(17) |
| C1B-O1B | 1.2660(11) | 1.2541(14) |
| C1B-C2B | 1.4350(13) | 1.4472(17) |
| C2B-C3B | 1.3727(13) | 1.3744(17) |
| C3B-C4B | 1.3916(13) | 1.3892(17) |
| C4B-C5B | 1.3789(14) | 1.3821(18) |
| C5B-C6B | 1.3763(13) | 1.3777(16) |
| C6B-C1B | 1.4343(13) | 1.4534(15) |
| C2B-N1B | 1.4594(12) | 1.4574(15) |
| C4B-N2B | 1.4471(12) | 1.4484(16) |
| C6B-N3B | 1.4538(13) | 1.4557(15) |
| N1B-O2B | 1.2206(14) | 1.2238(15) |
| N1B-O3B | 1.2253(14) | 1.2294(16) |
| N2B-O4B | 1.2319(14) | 1.2268(17) |
| N2B-O5B | 1.2261(13) | 1.2282(16) |
| N3B-O6B | 1.262(2) | 1.2293(13) |
| N3B-O7B | 1.223(3) | 1.2217(14) |
| O2A-C1A-O1A | 125.91(10) | 125.50(10) |
| O2A-C1A-C2A | 121.56(9) | 125.45(11) |
| O1A-C1A-C2A | 112.52(9) | 109.02(9) |
Table 2 Selected bond lengths (Å) and angles (°) in sarcosinium picrate (I) and betainium picrate (II).
| D-H···A | D-H | H···A | D···A | <DHA |
|---|---|---|---|---|
| (I) | ||||
| O1A-H1A···O1Bi | 0.81(2) | 1.78(2) | 2.5861(13) | 175(2) |
| N1A-H31A···O1Bii | 0.90 | 2.06 | 2.8261(13) | 142 |
| N1A-H32A···O2Aiii | 0.90 | 2.04 | 2.8034(13) | 142 |
| (II) | ||||
| O1A-H1A···O1Biv | 0.90(2) | 1.65(2) | 2.5265(13) | 163(2) |
Symmetry code: (i) –x+1, y–1/2, –z+1/2; (ii) –x+1, y+1/2, –z+1/2; (iii) –x+1, y+1/2, –z+1/2; (iv) –x+1, –y, –z+1.
Table 3 Hydrogen bonds parameters for sarcosinium picrate (I) and betainium picrate (II) sarcosinium picrate (I) and betainium picrate (II) (in Å and °).
As in some other cases [7-11] nitro groups neighboring to phenolic oxygen atoms in picrate anions are somewhat out of ring planes: torsion angles O2B-N1B-C2B-C1B and O7B-N3B-C6BC1B are equal to -49.9(1)° and -33.8(2)° respectively in (I) and 23.7(2)°, -25.6(2)° respectively in (II) compared to the third nitro groups: O4B-N2B-C4B-C3B 12.4(1)° in (I) and 4.0(2)° in (II).
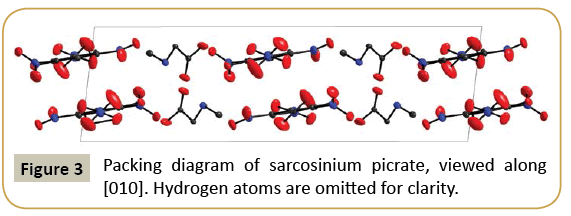
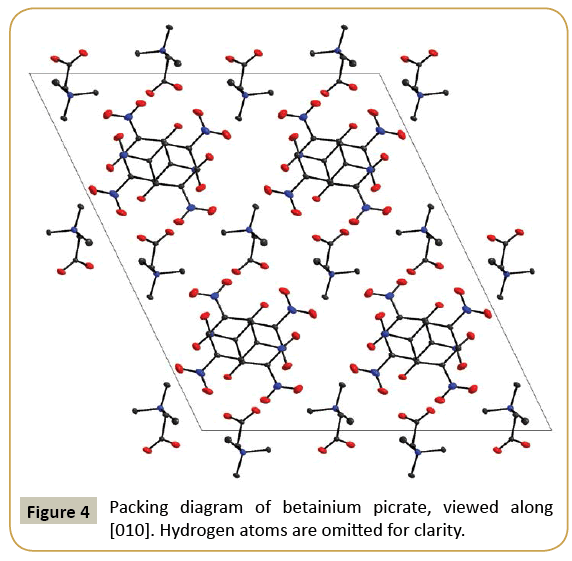
Although both sarcosinium picrate (I) and betainium picrate (II) crystallize in monoclinic symmetry, there are noteworthy differences in the packing of the units in both compounds. In (I), both picrate anions and sarcosinium cations are arranged in layers parallel (100), with alternating columns of cations and anions. In contrast, the structure of (II) consists of an arrangement of parallel picrate anions (all oriented parallel (010)) with Betainium cations in the interstices. These cations are not oriented parallel to the anions, but reach “up” and “down” so that the layers are interlocked. The packing diagrams of both compounds are shown in Figures 3 and 4, in the case of (I) viewed along a layer.
Vibrational spectra of sarcosinium picrate and betainium picrate
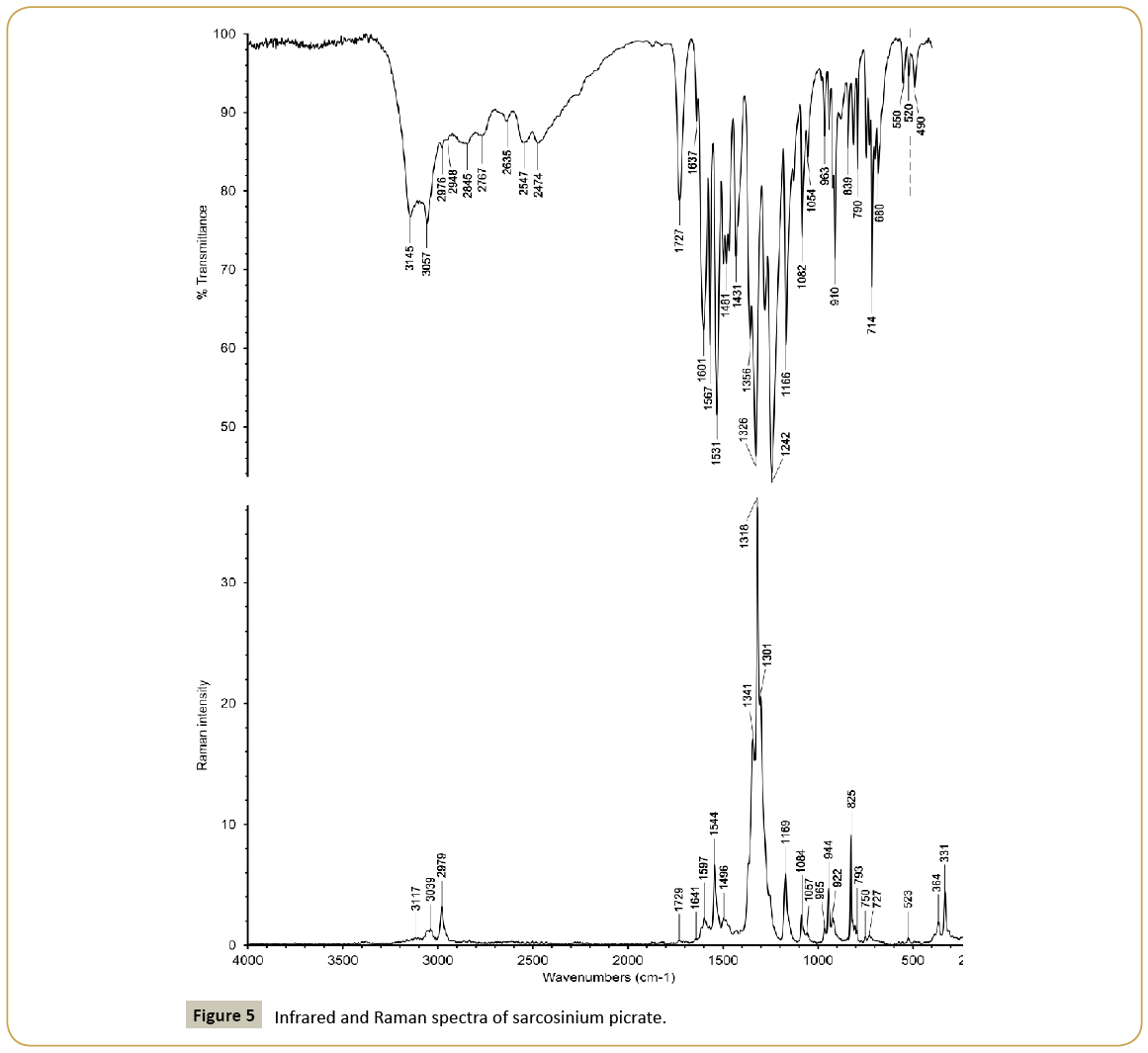
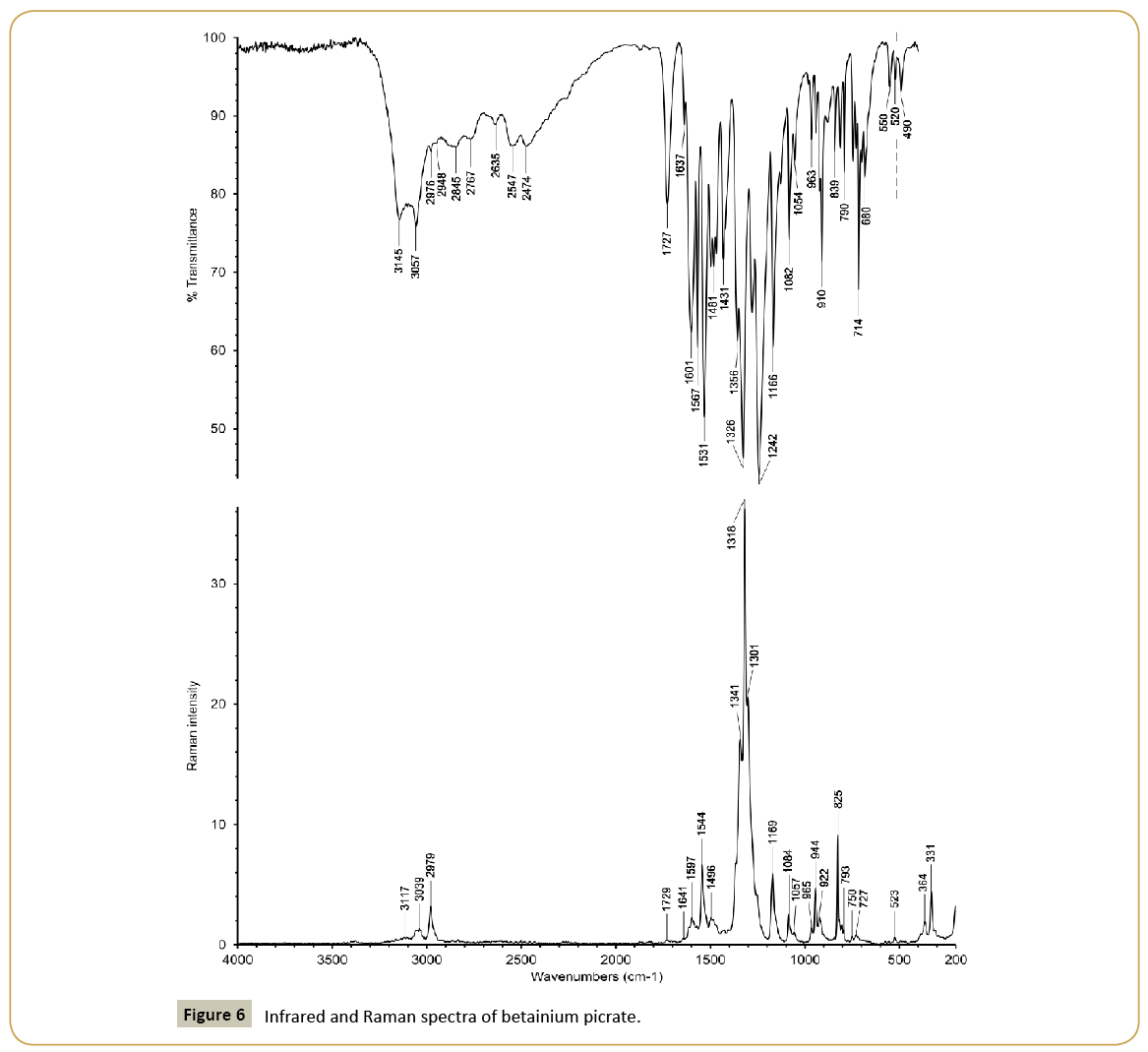
Infrared and Raman spectra of sarcosinium picrate (I) and betainium picrate (II) are shown in Figures 5 and 6 and wavenumbers of peaks and their assignments in Table 4. The spectra have some common features due to presence of picrate anions and common cationic functional groups (carboxyl groups and methyl groups) as well as distinctions caused by presence of NH2 + group in sarcosinium cation. The presence of carboxyl groups is reflected in presence of absorption bands at 1727 cm-1 (Figure 5) and 1728 cm-1 (Figure 6). Respective lines in the Raman spectra are very weak because of presence of very strong lines of symmetric stretching modes of nitro groups. Two other characteristic lines of nitro groups in the Raman spectra are asymmetric stretching and deformation scissoring modes at 1544 cm-1, 825 cm-1 (Figure 5) and 1545 cm-1, 825 cm-1 (Figure 6). Bands caused by asymmetric and symmetric modes of nitro groups are also among most strong absorption band in the IR spectra. The main distinction between sarcosinium and betainium cations, namely, the presence of NH2 + group is reflected in presence of the strong absorption band with peaks at 3145 cm-1 and 3057 cm-1 (Figure 5). The position of this band is in good agreement with that expected based on correlation between ν(N-H) and N···O distances [12]. In the IR spectrum of (II) (Figure 6) there are characteristic peaks of stretching vibrations of methyl groups of betainium cation in this region. So, it is possible that strong absorption band with peaks at 3145 cm-1 and 3057 cm-1 (Figure 5) is superposed on relatively weak peaks of stretching vibrations of methyl group. Expected position of ν(O-H) in the IR spectrum of (I) based on correlation between ν(O-H) and O···O distances [13] is ca. 2500 cm-1. So, the absorption band with peaks at 2547 cm-1 and 2474 cm-1 probably is a superposition of the ν(O-H) band and weak combination bands. In the structure of (II) there is only one hydrogen bond O1A-H1A···O1B with O···O distance equal to 2.5265(13) Å. Expected position of ν(O-H) for this hydrogen bond is ca. 1750 cm-1. However, instead of this there are absorption bands near 2496 cm-1 and 1860 cm-1 in the IR spectrum of (II) (Figure 6). We assume that these bands may relate to the hydrogen bond.
| (I) | (II) | Assignment | ||
|---|---|---|---|---|
| IR | Raman | IR | Raman | |
| 3145;3057 | 3117 | ν(NH) NH2+ | ||
| 3087;3069 | 3092;3072 | ν(CH) CH3 | ||
| 3049;3039 | 3040;3009 | 3051;3042;3010 | ν(CH) CH3 | |
| 2976;2948 | 2979 | 2974;2958 | 2981 | ν(CH) CH2 |
| 2845;2767;2635 | 2763 | Combi | ||
| 2547;2474 | 2492;2421 | 2618 | ν(OH), combi | |
| 1873;1823 | 1867 | 1862 | Combi | |
| 1727 | 1729 | 1728 | 1735 | ν(C=O) COOH |
| 1637 | 1641 | 1624 | ||
| 1601 | 1597 | 1605 | 1595 | ν(C=C) ring |
| 1567;1531 | 1544 | 1564;1538;1520 | 1572;1545;1518 | νas(NO2), δ(NH2+) |
| 1498 | 1496 | 1495 | 1496 | δas(CH3) |
| 1481;1467 | 1476 | 1473 | δ(CH2) | |
| 1431;1423 sh | 1425 | 1426 | 1446;1421 | |
| 1356;1326 | 1356;1341; 1318;1301 |
1385;1361; 1332;1311 | 1364; 1338;1313 | δs(CH3), νs(NO2) |
| 1279;1242 | 1255 sh | 1260;1225 | 1292;1275 | ν(C-O) ph,ν(C-OH) |
| 1207 | 1228 | |||
| 1166 | 1169 | 1160 | 1162 | δ(C-H) in-plane |
| 1126 | 1129 | |||
| 1082 | 1084 | 1085 | 1088 | τ(CH2) |
| 1054 | 1057 | 1051 | ν(C-N) | |
| 981;963 | 965 | 1003;989;959 | 965 | |
| 942 | 944 | 940 sh;930 | 945;936 | ν(C-C) |
| 921;910;879 | 922 | 910;889 | 913;891 | |
| 839 | 825 | 838 | 825 | δ(NO2) |
| 812;790 | 793 | 819;785;746 | 800;789;764 | δ(C-H) out-of-plane |
| 745;727;714;698 | 750;727 | 721;710;700 | 725 | ρ(CH2), ω(NO2) |
| 680;550;520 | 523 | 678;543;527;487 | 685;546 | ρ(NO2), ring breathing |
| 490 | 447;436;408 | |||
| 364;331 | 383;355;337 | |||
| 199;164 | 311;217 | |||
Symmetry code: (i) –x+1, y–1/2, –z+1/2; (ii) –x+1, y+1/2, –z+1/2; (iii) –x+1, y+1/2, –z+1/2; (iv) –x+1, –y, –z+1.
Table 4 Wavenumbers of peaks (cm-1) and assignment of some characteristic modes in the vibrational spectra of sarcosinium picrate (I) and betainium picrate (II).
Conclusions
In this work we have studied salt formation in the sarcosine-picric acid-water and betaine-picric acid-water systems in comparison with results of previously studied glycine-picric acid-water system. We found that in contrast with case of glycine, where the glycine glycinium picrate salt is formed at both 2:1 and 1:1 mole ratios, in cases of sarcosine and betaine simple salts of sarcosinium picrate and betainium picrate are formed at both 2:1 and 1:1 mole ratios. Crystal and molecular structures of obtained crystals have been determined and discussed as well as infrared and Raman spectra have been recorded and discussed based on structural data.
References
- Fleck M, Petrosyan AM (2014) Salts of amino acids: crystallization, structure and properties. Dordrecht: Springer.
- Ghazaryan VV, Fleck M, Petrosyan AM (2011) Glycine glycinium picrate – Reinvestigation of the structure and vibrational spectra. Spectrochim Acta 78: 128-132.
- NONIUS, Kappa CCD (1999) Program Package: COLLECT, DENZO, SCALEPACK, SORTAV, Nonius BV, Delft, The Netherlands.
- Otwinowski Z, Minor W (1997) Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol 276: 307-326.
- Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr 64: 112-122.
- Ichikawa M (1979) The effect of hydrogen bonding on the bond lengths and angles in carboxyl group. J Cryst Mol Struct 9: 87-105.
- Fleck M, Ghazaryan VV, Petrosyan AM (2012). β-Alaninium picrate: A new salt with di-β-alaninium dimeric cation. J Mol Struct 1019: 91-96.
- Ghazaryan VV, Fleck F, Petrosyan AM (2012) Structure and vibrational spectra of L-alanine L-alaninium picrate monohydrate. J Mol Struct 1015: 51-55.
- Zakharov BA, Ghazaryan VV, Boldyreva EV, Petrosyan AM (2015) L-serine picrates. J Mol Struct 1100: 255-263.
- Ghazaryan VV, Zakharov BA, Boldyreva EV, Petrosyan AM (2015). L-methioninium picrate. Spectrochim Acta 142: 344-349.
- Apreyan RA, Fleck M, Atanesyan AK, Sukiasyan RP, Petrosyan AM (2015). L-Nitroargininium picrate. J Mol Struct 1101: 236-242.
- Nakamoto K, Margoshes M, Rundle RE (1955) Stretching frequencies as a function of distances in hydrogen bonds. J Amer Chem Soc 77: 6480-6486.
- Novak A (1979) Vibrational spectroscopy of hydrogen bonded systems. In: Infrared and Raman Spectroscopy of Biological Molecules. NATO Advanced Study Institute Series 43: 279-283.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences