Quantitative Investigation of C-F...ÃÆà ½ÃâàInteraction in 4-(2-(((6-(trifluoromethyl) pyridin-2-yl)oxy)methyl)phenyl)-1,2-dihydro- 3H-pyrazol-3-one
Rahul Shukla, Chetan Shripanavar and Deepak Chopra
DOI10.21767/2470-9905.100028
Rahul Shukla1, Chetan Shripanavar2 and Deepak Chopra1*
1Crystallography and Crystal Chemistry Laboratory, Department of Chemistry, Indian Institute of Science Education and Research, Bhopal, Madhya Pradesh, India
2Department of Agrochemicals and Pest Management, Devchand College, Arjunnagar, Karnataka, India
- *Corresponding Author:
- Deepak Chopra
Crystallography and Crystal Chemistry Laboratory
Department of Chemistry
Indian Institute of Science Education and Research
Bhopal, Madhya Pradesh
India
Tel: +9107556691311
E-mail: dchopra@iiserb.ac.in
Received date: July 03, 2017; Accepted date: August 07, 2017; Published date: August 10, 2017
Citation: Shukla R, Shripanavar C, Chopra D. Quantitative Investigation of C-F···π Interaction in 4-(2-(((6-(trifluoromethyl) pyridin-2-yl)oxy)methyl)phenyl)-1,2-dihydro- 3H-pyrazol-3-one. Struct Chem Crystallogr Commun. 2017, 3:1. doi: 10.21767/2470-9905.100028
Abstract
In this study, we have investigated the nature and characteristics of C-F···π interactions present in the crystal structure of 4-(2-(((6-(trifluoromethyl)pyridin-2- yl)oxy)methyl)phenyl)-1,2-dihydro-3H-pyrazol-3-one (1). This is a case of an lp···π interaction where the lp of the fluorine interacts with the pyridinyl π-ring. The molecular pairs consisting of C-F···π interactions were stabilized with dispersion being the dominant force. The existence of these F···π interactions were further confirmed by the presence of (3,-1) bond critical points between fluorine and the carbon atoms of the π-rings.
Keywords
Molecular; Crystal structure; Carbon atoms
Introduction
Non-covalent interactions plays a very important role in molecular self-assembly and are very important aspect of supra-molecular chemistry [1,2]. In recent years, weak interactions involving organic fluorine have garnered significant attention due to its unique properties such as high electronegativity and small size [3,4]. It was previously believed that organic fluorine cannot participate in formation of hydrogen bond [5] but it has been now firmly established that fluorine can form highly directional and stabilized hydrogen bonds [6,7]. Apart from hydrogen bonds, fluorine can also participate in the formation of F···π interaction also where the lp of the fluorine interact with π-rings [8]. F···π interactions has been observed to play an important role in molecular crystals and hence it is of interest to quantitatively investigate the nature and characteristics of F···π interactions [9-12]. In this study, we have quantitatively investigate the role of C-F···π interactions in the crystal structure of 4-(2-(((6-(trifluoromethyl)pyridin-2-yl) oxy)methyl)phenyl)-1,2-dihydro-3H-pyrazol-3-one (1). The aim of this study was to evaluate the strength of the F···π interaction and their contribution towards molecular packing. Topological analysis was also performed in order to evaluate the magnitude of the electron density (ρ) and Laplacian (∇2ρ) at the bond critical point of the interacting atoms.
Experimental Details
The present compound [4-[2-({[6-(trifluoromethyl)pyridin- 2-yl]oxy}methyl)phenyl]-1,2-dihydro-3H-pyrazol-3-one] was synthesized by the process of reductive cyclization, using 0.367 gm, 0.001 mol of (2E)-3-methoxy-2-[2-({[6-(trifluoromethyl)pyridin-2- yl]oxy}methyl)phenyl]prop-2-enehydrazide, which is dissolved in 10 ml of methanol along with 2 drops of conc.H2SO4 (Scheme 1). The reaction mixture was refluxed for 6 h at 800C temperature and cooled to separate out the final product. The final product is again re-crystallized by dissolving in ethanol and by the process of slow evaporation needle shaped white transparent crystals of the product were obtained. The cyclization of hydrazide is a wellknown process and has been utilized extensively for synthesizing a variety of pyrazol derivatives [13,14].
X-ray data collection
Single crystal X-ray measurements were carried out on a Bruker AXS Kappa Apex 2 CCD diffractometer using monochromated Mo-Kα radiation (λ=0.71073 Å) in phi (φ) and omega (ω) scans at 100(2) K. Unit cell measurement, data collection, integration, scaling and absorption corrections were performed using Bruker Apex II software [15]. The intensity data were processed by using the Bruker SAINT [16] suite of programs. The crystal structure was refined by the full matrix least squares method using SHELXL14 [17] present in the program suite WinGX [18]. Empirical absorption correction was applied using SADABS [19]. The CF3 group is disordered over two independent orientations and refines in the ratio of 2:1. The non-hydrogen atoms were refined anisotropically and the hydrogen atoms bonded to C and N atoms were positioned geometrically and refined using a riding model with Uiso(H)=1.2Ueq (C, N). The molecular connectivity and the crystal packing diagrams were generated using the Mercury 3.9 (CCDC) program [20]. Geometrical calculations were done using PARST [21] and PLATON [22]. The details of data collection and crystal structure refinement are shown in Table 1.
| Empirical formula | C16H12F3N3O2 |
|---|---|
| Formula weight | 335.29 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a (Å) | 16.4436(17) |
| b (Å) | 18.236(2) |
| c (Å) | 10.4744(11) |
| α (°) | 90 |
| β (°) | 108.57(3) |
| γ (°) | 90 |
| V (A3) | 2977.3(5) |
| Z, Z¢ | 8, 2 |
| Dcalc (gcm-3) | 1.496 |
| µ (mm-1) | 0.126 |
| F (000) | 1376 |
| qmin, max | 2.588, 27.626 |
| Treatment of Hydrogens | Fixed |
| hmin,max, kmin,max,lmin,max | -21, 19, -23, 23, -13, 13 |
| No. of ref. | 24734 |
| No. of unique reflections/Observed reflections | 6067/3278 |
| Goodness-of-ÃÆïÃâìÃâÃÂt | 1 |
| Final R indices [I>2σ(I)] | R1 = 0.0965, wR2 = 0.2434 |
| R indices (all data) | R1 = 0.1750, wR2 =0.2793 |
| Largest difference peak and hole (e Å-3) | 0.55 and -0.52 |
| CCDC reference number | 1559231 |
Table 1: Single crystal data collection and refinement details of 1.
Theoretical calculations
Lattice energy and intermolecular interaction energies partitioned into coulombic (Ecoul), polarization (Epol), dispersion (Edisp), and repulsion energy (Erep) terms were evaluated using the PIXEL method present in the CLP module [23] which has been utilized in several similar studies. Crystal Explorer (version 17.5) software was also utilized for mapping 2D fingerprint plots [24] which helped in evaluating the contribution of different intermolecular interactions present in a molecule in the crystalline environment. Topological analysis was performed at B3LYP/6-311G** level of theory by using AIMALL software [25] which is based on Bader’s Quantum Theory of Atoms in Molecules [26]. For the purpose of quantitative analysis, fluorine atoms with major occupancy were considered for our calculations.
Results and Discussion
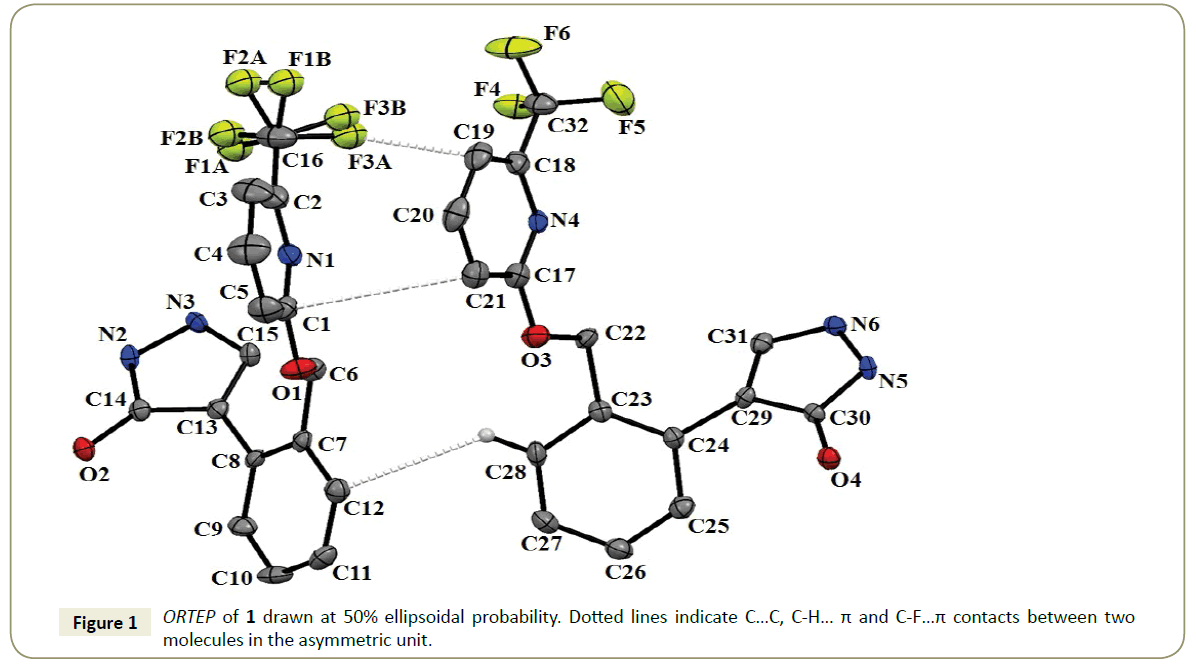
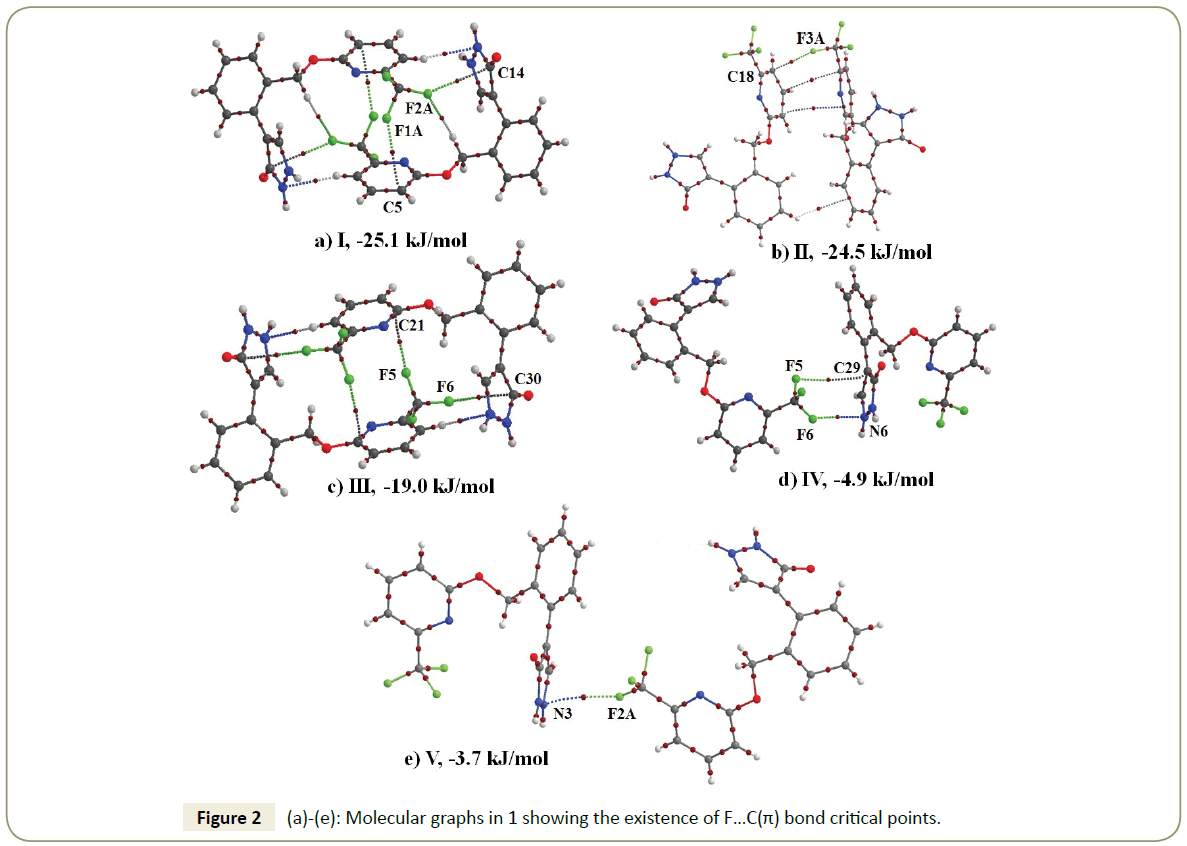
Figure 1 shows the ORTEP of the title molecule which crystallizes in monoclinic P21/c space group with Z=8. One of the molecules present in the asymmetric unit consisted of a disordered CF3 group, with the occupancy of the major: minor orientation being in the ratio of 0.57:0.43. The lattice energy of 1 was calculated to be -190.3 kJ/mol with dispersion being the largest contributor towards the stabilization (~42%) followed by stabilization from coulombic (~40%) and polarization (~18%) contribution (Table 2). Analysis of the crystal structure reveals that there are five different molecular motifs which are involved the formation of C-F···π interactions (Figure 2; Table 3). The interaction energies in these motifs ranged from -25.1 kJ/mol to -3.7 kJ/mol with dispersion being the largest contributor towards the stabilization (Table 3). The C-F···π(C) distance in these motifs were in the range of 2.93-3.77 Å while the C-F···π(C) directionality is in the range of 108-142°. It is important to note that in case of motif I, II and III that the total stabilization energy was significantly larger as compared to the other two motifs due to the presence of hydrogen bonds in addition to the F···π interaction which provided additional stability to the respective motifs (Table 3).
| Code | Ecoul | Epol | Edisp | Erep | Etot |
|---|---|---|---|---|---|
| 1 | -154.9 | -72.2 | -163.5 | 200.3 | -190.3 |
Table 2: Lattice Energy of 1 partitioned into different energy components (in kJ/mol)
| Motif | Centroid-Centroid Distance | Ecoul | Epol | Edisp | Erep | Etot | Symmetry | Interaction | Geometrical Parameters |
|---|---|---|---|---|---|---|---|---|---|
| (kJ/mol) | (kJ/mol) | (kJ/mol) | (kJ/mol) | (kJ/mol) | (Å/°) | ||||
| I | 5.869 | -1.1 | -11.8 | -52.3 | 40 | -25.1 | 2-x,1-y,-z | C16-F1A…C5 | 3.237/141 |
| C16-F2A…C14 | 3.438/141 | ||||||||
| C3-H3…N2 | 2.55/159 | ||||||||
| II | 6.362 | -5.4 | -3.6 | -40.6 | 25.1 | -24.5 | x,y,z | C16-F3A…C18 | 3.165/132 |
| C28-H28…C12 | 2.91/132 | ||||||||
| π(C1)…π(C21) | 3.464 | ||||||||
| III | 5.732 | 6.7 | -8.5 | -48.6 | 31.4 | -19 | 1-x,1-y,-z | C32-F6…C30 | 3.400/150 |
| C32-F5…C21 | 3.223/162 | ||||||||
| C19-H19…N6 | 2.63/163 | ||||||||
| IV | 10.444 | -0.2 | -1.2 | -9.4 | 5.9 | -4.9 | 1-x,-0.5+y,0.5-z | C32-F6…N6 | 2.932/137 |
| C32-F5…C29 | 3.777/107 | ||||||||
| V | 10.313 | 2.4 | -1.2 | -10.5 | 5.6 | -3.7 | 2-x,-0.5+y,0.5-z | C16-F2A…N3 | 3.031/142 |
Table 3: List of molecular motifs consisting of C-F···π interactions along with interaction energies and geometrical parameters.
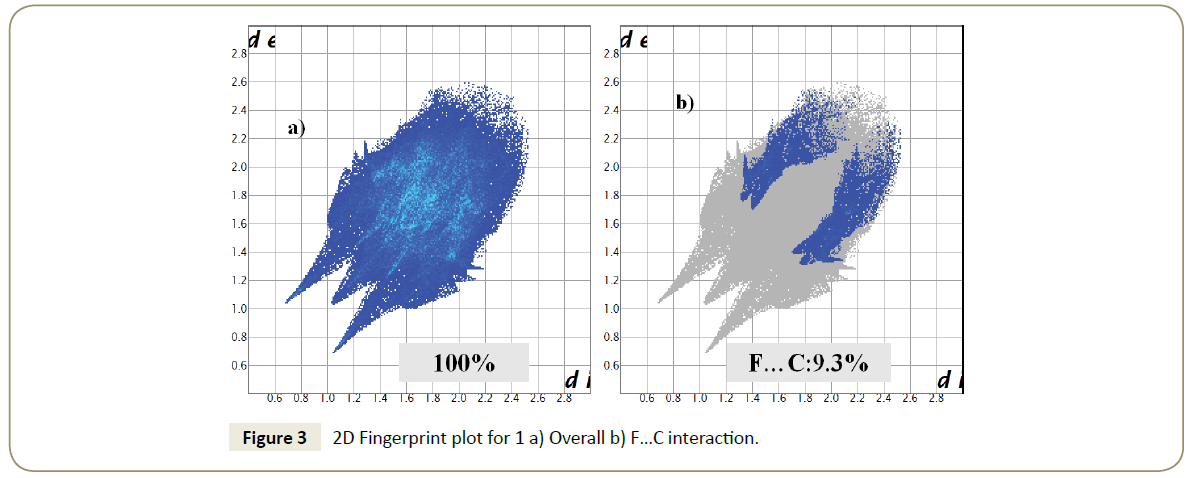
The presence of the F···π(C) interactions in the above discussed motifs was further confirmed by a topological analysis due to the presence of a bond critical point between the interacting fluorine and the carbon atom of the π-ring (Figure 2). The F…C bond path length in these ranged from 2.937Å to 3.916 Å. It is important to note that while the magnitude of the bond path length was similar to that of bond length; their origin and physical significance were different. The magnitude of ρ ranged from 0.013-0.055 e/ Å3 while the magnitude of ∇2ρ ranged from 0.199-0.797 e/Å5 (Table 4). In general, the magnitude of both ρ and ∇2ρ decreases with increasing value of BPL. The low value of ρ accompanied by positive value of Laplacian confirms F···C(π) interactions to be a case of closed-shell interactions. The Hirshfeld fingerprint plot also shows that F···C(π) interactions had 9.3% contribution towards the molecular packing and establish that F···π interaction indeed plays a very important role in the crystal packing of the title compound (Figure 3).
| Interaction | BPL (Å) | ρ (e/Å3) | Ñ2ρ (e/Å5) |
|---|---|---|---|
| C32-F6…N6 | 2.937 | 0.055 | 0.797 |
| C16-F2A…N3 | 3.056 | 0.046 | 0.697 |
| C32-F5…C21 | 3.198 | 0.038 | 0.557 |
| C16-F1A…C5 | 3.266 | 0.036 | 0.518 |
| C32-F6…C30 | 3.409 | 0.018 | 0.301 |
| C16-F2A…C14 | 3.445 | 0.017 | 0.290 |
| C16-F3A…C18 | 3.784 | 0.043 | 0.555 |
| C32-F5…C29 | 3.916 | 0.013 | 0.199 |
Table 4: Topological parameters obtained for different C-F…π(C) interactions present in 1.
Summary
In this study, we have quantitatively investigated the role of C-F…π(C) interactions in the crystal structure of 4-(2-(((6-(trifluoromethyl)pyridin-2-yl)oxy)methyl)phenyl)-1,2- dihydro-3H-pyrazol-3-one. It was evident that both dispersion and coulombic energy components had significant contribution towards the crystal lattice. Calculations on the interaction energy reveal that the motifs consisting of C-F…π interactions were attractive in nature with dispersion being the dominant contributor towards stabilization. Topological calculations further confirmed the existence of these weak interactions. Future study will be focussed on analysing the interplay between C-F…π and other non-covalent interactions, such as hydrogen bonds, present in the crystal structure of related molecules and their subsequent role in formation of the 3D molecular packing.
Acknowledgements
RS thanks DST for INSPIRE-PhD Fellowship. DC thanks IISER Bhopal for infrastructural and research facilities.
References
- Bombicz P, Gruber T, Fischer C, Weber E, Kálmán A (2014) Fine tuning of crystal architecture by intermolecular interactions: synthon engineering. CrystEngComm 16: 3646-3654.
- Desiraju GR (2000) Hydrogen bonds and other intermolecular interactions in organometallic crystals. J ChemSoc Dalton Trans: 3745-3751.
- Chopra D, Guru Row TN (2011) Role of organic fluorine in crystal engineering. CrystEngComm 13: 2175-2186.
- Chopra D (2012) Is Organic Fluorine Really “Not” Polarizable? Cryst Growth Des 12: 541-546.
- Dunitz JD,Taylor R (1997) Organic Fluorine Hardly Ever Accepts Hydrogen Bonds. ChemEur J 3: 89-98.
- Thakur TS, Kirchner MT, Bläser D, Boese R,Desiraju GR (2010) C–HÃÆâÃâââ¬Â¹ÃâïF–C hydrogen bonding in 1,2,3,5-tetrafluorobenzene and other fluoroaromatic compounds and the crystal structure of alloxan revisited. CrystEngComm 12: 2079-2085.
- Shukla R, Chopra D (2015) Crystallographic and computational investigation of intermolecular interactions involving organic fluorine with relevance to the hybridization of the carbon atom. CrystEngComm 17: 3596-6309.
- Holl MG, Struble MD, Singal P, Seigler MA,Lectka T (2016) Positioning a Carbon–Fluorine Bond over the πÃÆâÃâââ¬Ãâââ¬Â¦Cloud of an Aromatic Ring: A Different Type of Arene Activation. AngewChemInt Ed 55: 8266-8269.
- Panini P, Chopra D (2012) Role of intermolecular interactions involving organic fluorine in trifluoromethylatedbenzanilides. CrystEngComm 14: 1972-1989.
- Lu YX, Zou JW, Wang YH, Yu QS (2007) Substituent Effects on Noncovalent Halogen/π Interactions: Theoretical Study. Int J Quant Chem 107: 1479-1486.
- Li P, Maier JM, Vik EC, Yehl CJ, Dial BE, et al. (2017) Stabilizing Fluorine– π Interactions. AngewChemInt Ed 56:1-5.
- Scerba MT, Bloom S,Haselton N, Siegler M, Jaffe J, et al.(2012) Interaction of a C−F Bond with the π-System of a C=C Bond or “Head On” with a Proximate C−H Bond. J Org Chem 77:1605-1609.
- Burbuliene MM, Bobrovas O,Vainilavicius P (2006) Synthesis and intramolecular cyclization of 2-methylsulfany-4-oxo-3(4H)-quinazolinyl)acetohydrazide. J Heterocyclic Chem 43: 43-47.
- Majumdar P, Pati A, Patra M, Behera RK, Behera AK (2014) Acid Hydrazides, Potent Reagents for Synthesis of Oxygen-, Nitrogen-, and/or Sulfur-Containing Heterocyclic Rings. Chem Rev 114: 2942-2977.
- Bruker Analytical X-ray Systems (2006) Apex2, Version 2 User Manual, M86-E01078, Madison, WI.
- Siemens Analytical X-ray Instruments Inc (1995) Siemens, SMART System, Madison, MI.
- Sheldrick G M (2008) A short of SHELX.ActaCrystallogr Sect. A: FoundCrystallogr64: 112-122.
- Farrugia LJ (1999) WinGXsuite for small-molecule single-crystal crystallography. J ApplCrystallogr 32: 837-838.
- Sheldrick GM (2007) SADABS, Bruker AXS, Inc. Madison, WI.
- Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, et al. (2008) Mercury CSD 2.0 - New Features for the Visualization and Investigation of Crystal Structures. J ApplCryst 41: 466-470.
- Nardelli M (1995) PARST95-an update toPARST: a system of Fortran routines for calculating molecular structure parameters from the results of crystal structure analyses. J ApplCryst 28: 659-659.
- Spek AL (2009) Structure validation in chemical crystallography. ActaCrystD 65: 148-155.
- Dunitz JD,Gavezzotti A (2012) Supramolecularsynthons: validation and ranking of intermolecular interaction energies. Cryst Growth Des 12: 5873-5877.
- Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, et al. (2017) CrystalExplorer17.
- Keith TA (2013) AIMALL, version 13.05.06, TK Gristmill Software, Overland Park, KS, USA.
- Bader R (1990) Atoms in Molecules: A Quantum Theory, International Series of Monographs on Chemistry. Clarendon Press.
s
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences