Electrons Charge Magnitudes (Ecm) are Dissimilar for Different Mass Atoms
Abu Mohammad Azmal Morshed*
Abu Mohammad Azmal Morshed*
Department of Chemistry, Bangladesh University of Textiles, Tejgaon, Dhaka
*Corresponding author: Morshed AME, Department of Chemistry, Bangladesh University of Textiles, Tejgaon, Dhaka; Tel No: 88-01674643058; E-Mail: azmal.morshed@gmail.com
Received date: July 18, 2020; Accepted date: August 20, 2021; Published date: August 30, 2021
Citation: Morshed AME (2020) Electrons Charge Magnitudes (Ecm) are Dissimilar for Different Mass Atoms. Chem Crystallogr Commun Vol: 7 No: 4.
Abstract
Relations of quantities such as charge and energy are correlated to mass. Electrical charge does not exist without mass; actually, charge might be called a property of mass which in itself is a form of energy, as exposed by Einstein. Different atoms of different elements contain different atomic size and mass, so all electrons of different mass atoms couldn't have the same electron charge magnitudes (ECM). The different atoms with different atomic mass (different matters atoms) contain different electron charge magnitudes. The new assumptions of the concept of Electron Charge Magnitudes (ECM): Electron Charge Magnitudes are different for different mass atomic electrons. Bigger the atomic mass (size) bigger is the electrons charge magnitudes”. There is relation regarding electrostatic force among the electron charge magnitudes may be called as electrons charge magnitudes electromagnetic theory (ECMET) as: There are fascinations (weak interaction) between the electrons with different electron charge magnitudes and repulsion only between the electrons with the same charge magnitude.
Keywords: Charge, Magnitudes, Quantum, Charge, Mechanics, electrons, etc.
Introduction
Atoms are considered the basic unit of matter because atoms construct up the elements which in turn combine to form compounds that exist in all states1,2. Electrons are subatomic particles with a negative elementary charge3,4. Electrons are said to be elementary particles because they dont have any known substructures or components yet. The elementary particles are what create atoms, and the other elementary particles the particles known as fermions. Fermions include leptons, bosons, and quarks5,6. These particles are what make up everything that we know in the universe. The charge is a property of matter: just like mass, volume, or density which is measurable7. Electric charge is the physical property of matter that causes it to experience a force when placed in an electromagnetic field8. Knowledge of chemistry of matters, compounds has reached its almost final developed stage, but still few basic questions dont find true answer because of some deficiency in the atomic model description. One of the most important questions is: “why the electron charge density increases at the juncture of bonded atoms? which domino effect the chemical bond strength by increasing the bond dissociation constants (pka value for acids). The assumptions of new ECM concepts will able to take away many contradictions of atomic electrostatic properties. This is first time in the history of Quantum Mechanics the new concepts of Electrons Charge Magnitudes (ECM) introduced. It is firmly believed that the new concepts will bring a revolutionary contribution on the knowledge of atomic structures electronic configuration, chemical bonding, molecules formation, MOT, VBT, chemical reactivity and compounds properties etc.
Discussion
The key concept atomic charge has been described by two types: positive (+) charge carried by the protons arranged at the center of the atoms and negative (-) charges carried by the electrons distributed among different energy shells around the centre of the atoms i.e., Nucleus. The positive charge on a proton is equal in magnitude to the negative charge on an electron of same atoms. As a result, a neutral atom carries an equal number of protons and electrons. It is considered that all the electrons and protons carry the same amount of charge, just different in type (+ve & -ve) is true for same element’s atoms (contain same atomic mass) but different for different mass atoms according my concept (Azmal's concepts for electron charge magnitudes).
It is known that the charge of any components depends on different mass of elements, so all atoms electrons cannot have the same charge magnitudes. All the atoms of different matters are not same by size and mass. A different atom varies with thedifferent atomic size (radius)/mass, so the electron charge magnitudes (ECM) cannot be the same. According to my perception the electrons charge magnitudes (ECM) are different by area of magnitudes for different mass atoms electrons which depend mainly on three variables as: 1. Atomic size/radius. 2. Mass of the atoms (the mass is directly related to the charge of the components), and 3.Electromagnetic properties of atom (electropositive & electronegative nature).
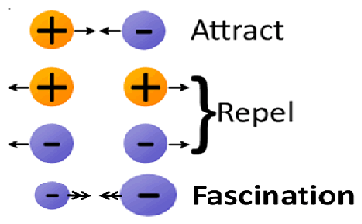
The electrons charge magnitudes (ECM) are different for differentelements atoms electrons because of difference in atomic size, atomic mass and electromagnetic nature.The charge ofproton and electron are exactly the same size but opposite for a similar type of atomthe charge magnitude of different atoms electrons. The charge magnitudes are always same for all electrons, protons of same atom which provide neutral atomic body. The charge magnitude of all electrons is same only among same atomic electronsbut contain different charge magnitudes in different atom's electrons. The different charges magnitude of different element's electrons can be quantified from the different atomic mass. There are fascinations (week attraction force) among the neighbor different electron charge magnitudes (Figure-2).
The new ECM concept illustrate that, electrons charge magnitudes are same only for the same atomic mass electrons but contains identical difference in size of magnitudes with the electrons of different mass atoms i.e., different atoms electrons change magnitudes are different in size. There are definite fascinations (week attraction force) between the electrons with different charge magnitudes of different mass atoms. The attraction force is because of the electrons with bigger electron charge magnitudes of big atoms contain more electro negativity then electrons with small electron charge magnitudes of small mass atom. The difference in electrons charge magnitudes causes the fascination between the same charged (-ve) electrons containing different electrons charge magnitudes of different mass atoms. The electromagnetic nature of different electrons charge magnitudes is deduced under Electrons Charge Magnitudes Electromagnetic Theory (ECMET)” as: There are fascinations (weak attraction force) between the electrons with different electrons charge magnitudes but execute repulsion among the electrons with same charge magnitudes.
The assumptions of Electrons Charge Magnitudes (ECM) are as follows ( Azmals concept):
Electron charge magnitudes (ECM) are same only for the electrons of the same mass atoms' but Electrons charge magnitudes (ECM) are different for different size(mass) atoms electrons i.e., ECMare bigger for big(mass) atoms' electrons and small for the smaller size(mass) atoms' electrons.
Electrons charge magnitudes (ECM) are usually bigger for the electronegative atoms electrons then electropositive atoms electrons. Electrons charge magnitude’s natures are different for electropositive and electronegative atoms electrons.
Conclusions
The new concept of the electrons charge magnitude(ECM) will assist the world to open new windows of knowledge of understanding about different types of chemical bonding, bond strength, electrons charge density (electrons cloud), exact reasons of polarity in compounds, molecule and atoms. The concept is able to explain the reasons of different reactivities of atoms, molecules and compounds. The new concept is also capable toanswer many unresolved problems in the atomic charge concept, ionization potential, causes of unusual properties in chemical bodings and can elucidate the properties of compounds. I am hopeful that the new electrons charge magnitudes (ECM)concept will introduce new horizon in the quantum mechanics acquaintance.
References
- Demtröder, (2002) Atoms, Molecules and Photons an Introduction to Atomic- Molecular- and Quantum Physics. 1st ed. Springer.
- Elizabeth. Patterson, John Dalton, (1970) the Atomic Theory, Garden City, NY: Doubleday.
- Y. Herron, (1951) An atomic structure model, J. Chem. Educ., 28(9), p-473.
- A.Cotton, (1990) Chemical Applications of Group Theory, 3 rd ed.; Wiley New York.
- A. V. Ebsworth, D. W. H. Rankin, and S. Cradock (1991) Structural Methods in Inorganic Chemistry, 2nd ed.; CRC Press: Boca Raton FL.
- E. Housecroft and A. G. Sharpe, Inorganic Chemistry, 4th Ed, Pearson, 2012.
- Petrucci, W.Harwood, G. Herring, and J.Madura, (2007) General Chemistry. 9th ed. Upper Saddle River, New Jersey: Pearson Prentince Hall.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences