Crystal Structure of Mixed-metal Phosphite, Pb2Ga(HPIIIO3)3(PVO3)
Jun-Ling Song1 and Xi-Jia Wang2
Jun-Ling Song1* and Xi-Jia Wang2
1China-Australia Joint Research Centre for Functional Molecular Materials, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China
2State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, People's Republic of China
- *Corresponding Author:
- Song JL
China-Australia Joint Research Centre for Functional Molecular Materials, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China
Tel: +8659183736672
E-mail: s070054@e.ntu.edu.sg
Received Date: December 18, 2015 Accepted Date: January 07, 2016 Published Date:J anauary 12,2016
Abstract
One mixed-metal phosphite namely, Pb2Ga(HPO3)3(PO3) (Compound 1) was obtained by a facile hydrothermal method and structurally characterized. Single crystal X-ray diffraction analysis reveals that crystallizes in the orthorhombic, space group Cmcm with unit cell dimensions, a=5.2572(12) Å, b=18.505(5) Å, c=12.544(3) Å and α=β=γ=90°. The crystal structure of Pb2Ga(HPO3)3(PO3) exhibits a complicated 3D framework based on PbO6 and GaO6 octahedral connected by HPO3 and H2PO3 anions via corner- or edge-sharing
https://betsatgirisi.com https://bettilte.com https://vegabete.com https://kanyongiris.com https://matgiris.com https://celtabetegiris.com https://hilbetegiris.com https://melbete.com https://kinbettinge.com https://wipbett.com https://pusulabetegiris.com https://superbahiss.com https://lidyagiris.com https://holiganbete.com https://1xbetgiriss.com https://asyabahise.com https://jetbahise.com https://betdoksana.com https://betebetle.com https://betgramagiris.com
Keywords
Crystal structure; Hydrothermal reactions; Mixed-metal phosphites; 3D framework; Single-crystal X-ray diffraction
Introduction
Metal phosphites have been paid more and more attention because of their abundant structures and potential applications in sorption, magnetism, photocatalyst and nonlinear optics [1-10]. Especially, pure inorganic metal phosphites with good thermostability and chemical stability have been exploited their application in various fields [9-13]. For example, two maingroup metal phosphites, namely, RbIn(HPO3)2 [9] and SnHPO3 [10], exhibit second harmonic generation (SHG) responses. Two alkali transition metal phosphites, namely, Li3Fe2(HPO3)3Cl and Li1.43[FeII 4.43FeIII 0.57(HPO3)6]1.5H2O, are good candidates of cathode materials [11,12]. In addition, a series of transition-metal (TM) phosphites exhibit different magnetic properties, such as Na2M(HPO3)2 (M=Ni, Fe, Co) and A [M(HPO3)2] (A=NH4, K, Rb and M=V, Fe) [13,14]. The above-mentioned compounds display different properties with amazing structures due to the different connectivity modes between metal ions and phosphite anions. Thus, it is of vital importance and urgent to explore the structure of phosphite compounds to understand the relationship in the above mentioned properties. In view of the above mentioned discussion, new mixed-metal phosphite should been prepared and study the relationship between the crystal structural and the properties. Thus, here, we prepared the synthesis and crystal structure one new mixed-metal phosphite, namely, Pb2Ga(HPO3)3(PO3).
Experimental
The starting materials were purchased from the Shanghai Reagent Factory (AR, 99.0%) without further purification.
Synthesis of Pb2Ga(HPO3)3(PO3)
A mixture of PbO (1 mmol), Ga2O3 (0.3 mmol), H3PO3 (2.5 mmol) and H2O (5 mL) was sealed in an autoclave equipped with a Teflon liner (25 mL) and heated at 180°C for three days followed by being slowly cooled to room temperature at a rate of 5°C/h. The initial and final pH values of the solution were about 0.5 and 1, respectively. Single crystals suitable for X-ray diffraction studies were obtained and the product was colorless prism-shaped crystals.
Data collection
X-ray diffraction data collection for Pb2Ga(HPO3)3(PO3) was performed on a Rigaku Mercury CCD diffractometer with Mo-Kα radiation (λ=0.71073 Å) at 293(2) K. The data sets were corrected for Lorentz and polarization factors as well as absorption by the multi-scan method [15-17]. The structure was solved by the direct method and refined by full-matrix least-squares fitting on F2by SHELX-97 [18,19]. All non-hydrogen atoms were refined with anisotropic thermal parameters. According to the charge balance and bond valence calculations, all the H atoms in Pb2Ga(HPO3)3(PO3) were needed and assigned to P-H bonds, but they were not refined due to the difficulty in the determination of their precise locations. All atoms were refined with anisotropic thermal parameters except for O(1) and O(2) in Pb2Ga(HPO3)3(PO3), which was refined with ‘isor’ instruction. If this constraint is not applied, the displacement parameters for O(1) and O(2) will be abnormally small. The P(2) atoms in this compound is disordered with the P(2)-P(2)’ distance of 0.889(12) Å. And the structure was also checked for possible missing symmetry with PLATON. Crystallographic data and structural refinements are summarized in Table 1. Atomic coordinates (× 104) and equivalent isotropic displacement parameters, important bond distances and angles are listed in Tables 2 and 3, respectively.
| Compound | 1 |
|---|---|
| Empirical formula | Pb2Ga(HPO3)3(PO3) |
| Formula weight | 833.97 |
| Temperature | 293(2) K |
| Crystal system | Orthorhombic |
| Space group | Cmcm |
| a/Å | 5.2572(12) |
| b/Å | 18.505(5) |
| c/Å | 12.544(3). |
| V/Å3 | 1220.3(5) |
| Z | 4 |
| Dc/g.cm-3 | 4.539 |
| m(Mo Kα)/mm-1 | 30.432 |
| F(000) | 1484 |
| Reflections collected/unique | 5010/809 [R(int)=0.0602] |
| Completeness to theta=27.46 | 99.4 % |
| Data/restraints/parameters | 809/6/65 |
| Goodness-of-fit on F^2 | 1.183 |
| Final R indices [I>2sigma(I)]a | R1=0.0333, wR2=0.0686 |
| R indices (all data) | R1=0.0355, wR2=0.0696 |
| Extinction coefficient | 0.00091(9) |
| Largest diff. peak and hole | 1.813 and -1.714 e. Å-3 |
aR1=Σ||Fo|-|Fc||/Σ|Fo|; ωR2={Σω[(Fo)2 (Fc)2]2/Σω[(Fo)2]2}1/2
Table 1: Crystal data and structural refinement for Pb2Ga2(HPO3)3(H2PO3).
| Atoms | x | y | z | U(eq) |
|---|---|---|---|---|
| Pb(1) | 5000 | 1900(1) | 1004(1) | 15(1) |
| Ga(1) | 5000 | 0 | 5000 | 8(1) |
| P(1) | 0 | 2841(2) | 2500 | 12(1) |
| P(2) | 4155(11) | 200(3) | 2500 | 12(1) |
| P(3) | 5000 | 3924(1) | 190(2) | 9(1) |
| O(1) | 0 | 3338(8) | 3470(11) | 11(3) |
| O(2) | 2370(19) | 2403(5) | 2500 | 33(2) |
| O(3) | 5000 | 987(6) | 2500 | 18(2) |
| O(4) | 5000 | -231(4) | 3470(6) | 16(2) |
| O(5) | 2581(10) | 4260(3) | -269(4) | 14(1) |
| O(6) | 5000 | 3119(4) | -65(7) | 19(2) |
Table 2: Table 2 Atomic coordinates (× 104) and equivalent isotropic displacement parameters (Å2 × 103) for the non-hydrogen atoms of Pb2Ga(HPO3)3(H2PO3).
| Pb(1)-O(2)#1 | 2.510(6) | Ga(1)-O(5)#5 | 1.957(5) |
|---|---|---|---|
| Pb(1)-O(2) | 2.510(6) | Ga(1)-O(5)#6 | 1.957(5) |
| Pb(1)-O(3) | 2.525(7) | Ga(1)-O(5)#7 | 1.957(5) |
| Pb(1)-O(6) | 2.624(7) | Ga(1)-O(4) | 1.966(7) |
| Pb(1)-O(5)#2 | 2.702(5) | Ga(1)-O(4)#8 | 1.966(7) |
| Pb(1)-O(5)#3 | 2.702(5) | P(1)-O(2)#9 | 1.486(9) |
| Ga(1)-O(5)#4 | 1.957(5) | P(1)-O(2) | 1.486(9) |
| P(1)-O(1) | 1.525(14) | P(3)-O(5)#1 | 1.528(5) |
| P(1)-O(1)#10 | 1.525(14) | P(2)-O(4)#10 | 1.521(8) |
| P(3)-O(6) | 1.525(7) | P(2)-O(4) | 1.521(8) |
| P(3)-O(5) | 1.528(5) | P(2)-O(3) | 1.522(12) |
| O(2)#1-Pb(1)-O(2) | 66.9(4) | O(5)#4-Ga(1)-O(5)#5 | 87.8(3) |
| O(2)#1-Pb(1)-O(3) | 72.1(2) | O(5)#4-Ga(1)-O(5)#6 | 180.0(2) |
| O(2)-Pb(1)-O(3) | 72.1(2) | O(5)#5-Ga(1)-O(5)#6 | 92.2(3) |
| O(2)#1-Pb(1)-O(6) | 93.6(2) | O(5)#4-Ga(1)-O(5)#7 | 92.2(3) |
| O(2)-Pb(1)-O(6) | 93.6(2) | O(5)#5-Ga(1)-O(5)#7 | 180.000(1) |
| O(3)-Pb(1)-O(6) | 162.8(3) | O(5)#6-Ga(1)-O(5)#7 | 87.8(3) |
| O(2)#1-Pb(1)-O(5)#2 | 145.7(2) | O(5)#4-Ga(1)-O(4) | 90.9(2) |
| O(2)-Pb(1)-O(5)#2 | 105.9(3) | O(5)#5-Ga(1)-O(4) | 90.9(2) |
| O(3)-Pb(1)-O(5)#2 | 73.85(19) | O(5)#6-Ga(1)-O(4) | 89.1(2) |
| O(6)-Pb(1)-O(5)#2 | 120.56(19) | O(5)#7-Ga(1)-O(4) | 89.1(2) |
| O(2)#1-Pb(1)-O(5)#3 | 105.9(3) | O(5)#4-Ga(1)-O(4)#8 | 89.1(2) |
| O(2)-Pb(1)-O(5)#3 | 145.7(2) | O(5)#5-Ga(1)-O(4)#8 | 89.1(2) |
| O(3)-Pb(1)-O(5)#3 | 73.85(19) | O(5)#6-Ga(1)-O(4)#8 | 90.9(2) |
| O(6)-Pb(1)-O(5)#3 | 120.56(19) | O(5)#7-Ga(1)-O(4)#8 | 90.9(2) |
| O(5)#2-Pb(1)-O(5)#3 | 60.3(2) | O(4)-Ga(1)-O(4)#8 | 180.000(1) |
| P(2)#1-P(2)-O(4)#10 | 73.0(2) | O(2)#9-P(1)-O(2) | 113.9(9) |
| P(2)#1-P(2)-O(4) | 73.0(2) | O(2)#9-P(1)-O(1) | 109.2(3) |
| O(4)#10-P(2)-O(4) | 106.2(6) | O(2)-P(1)-O(1) | 109.2(3) |
| P(2)#1-P(2)-O(3) | 73.0(2) | O(2)#9-P(1)-O(1)#10 | 109.2(3) |
| O(4)#10-P(2)-O(3) | 114.6(4) | O(2)-P(1)-O(1)#10 | 109.2(3) |
| O(4)-P(2)-O(3) | 114.6(4) | O(1)-P(1)-O(1)#10 | 105.9(11) |
| O(6)-P(3)-O(5) | 108.6(3) | O(6)-P(3)-O(5)#1 | 108.6(3) |
| O(5)-P(3)-O(5)#1 | 112.7(4) |
Table 3: Bond distances (Å) and angles [o] for Pb2Ga(HPO3)3(PO3).
Results and Discussion
Structure solving and refinement
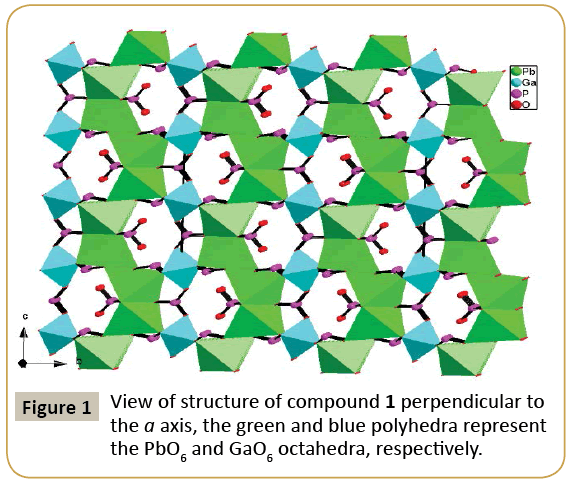
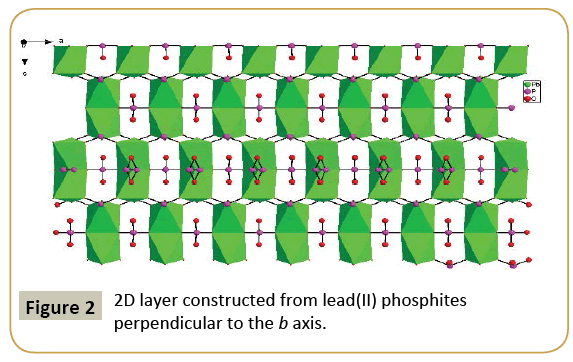
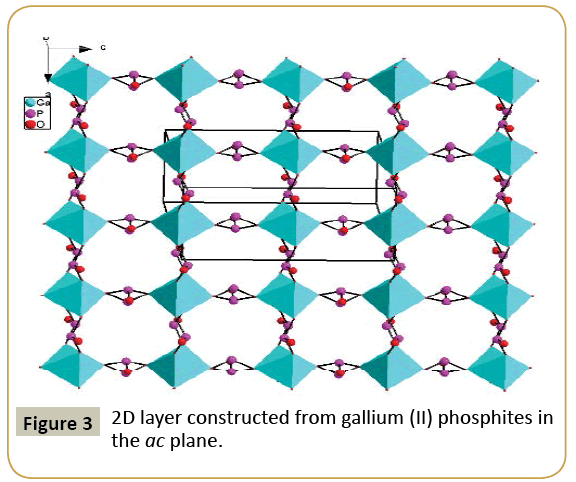
Compound 1, namely, Pb2Ga(HPO3)3(PO3), crystallizes in the orthorhombic space group Cmcm (no. 63) and features a 3D framework formed by the interconnection of 2D layer of lead phosphites and gallium phosphites (Figure 1). The asymmetric unit of Pb2Ga(HPO3)3(PO3) includes 11 independent non-H atoms, including one Pb, one Ga, three P and six O atoms, in addition, all atoms except O(5) lie in special positions. Pb(1) is six coordinated by six oxygens from five HPO3 anions and one P(1)O4 anion in a distorted pentagonal pyramid geometry, Ga(1) is also six coordinated by six oxygens from six HPO3 anions in a octahedral geometry. And the Pb2+ ions shows stereochemically active lone pairs. The Pb−O and Ga-O distances range from 2.510(6) to 2.702(5) Å, 1.957 (5) to 1.966 (7) Å, respectively. All of these bond distances are comparable to those reported for other lead(II) phosphites [20] and gallium phosphites [9,21]. The calculated total bond valences for Pb(1), Ga(1) and P(1) atoms are 1.671 3.025 and 5.430, respectively, indicating that Pb, Ga and P(1) are in oxidation states of +2, +3 and +5, respectively, in accordance with the crystal structure results. P(1) atom is coordinated with two O(1) and two O(2) atoms to form [PO4]3− tetrahedral units, and the occupancy of P(1), O(1) and O(2) is 25%, 25% and 50%, respectively, while P(2) and P(3) atoms bond to three oxygen atoms and one hydrogen atom, forming the pseudo-tetrahedral coordination geometry. The presence of P(1)O4 phosphates might be due to the H3PO3 is oxidized in the reaction progress. The P−O bond distances range from 1.486 (9) to 1.528(5) (Table 2). The phosphates and phosphites anions adopt different coordination modes. P(1)O4 phosphates shows a μ4-η2η2-coordination mode that bridges 4Pb atoms with two O atoms, HP(2)O3 anion displays 4.211 coordination mode that bridges 4Pb atoms, and HP(3)O3 anion adopts 5.221 coordination mode that bridges 5Pb atoms. The PbO6 octahedra and HPO3 and PO4 anions are interconnected via edge-sharing into a novel 2D anionic network in the ac palne with the 6-member rings tunnels along b-axis (Figure 2). While, the GaO6 octahedra and HPO3 pseudo-tetrahedra are interconnected via vertex-sharing resulting in a 2D anionic network in the ac palne with the 4-Member Rings tunnels along b-axis (Figure 3). Such two type of 2D layers further bridges by corner-sharing oxygen atom to yield a 3D framework in the ab plane (Figure 1).
.
Conclusions
In summary, we reported one new mixed-metal phosphites, namely, Pb2Ga(HPO3)3(PO3) using a facile hydrothermal method and it crystallizes in the orthorhombic space group Cmcm, which features 3D framework formed by the interconnection of 2D layer of lead(II) phosphites and 2D layer of gallium(III) phosphites. Our future research efforts will be devoted to the design and preparation of different mixed-metal phosphites with d0-transition metals such as Ti4+, V5+ and Mo6+ to exploit this related systems and study the relationship between the crystal structures and properties of this related materials.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21403232) and the Fundamental Research Funds for the Central Universities (No. JUSRP11518).
Additional Information
Crystallographic data for the structures reported here can be obtained from the FIZ Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany, Fax: +497247808666; E-mail: crysdata@fiz-karlsruhe. de on quoting the depository numbers CSD 430503.
References
- Lin HY, Wang SL (2013) Crystalline Inorganic Frameworks with 56-Ring, 64-Ring, and 72-Ring Channels. Science 339: 811-813.
- Krivovichev SV, Mentre O (2013) Anion-Centered Tetrahedra in Inorganic Compounds. Chem Rev 113: 6459-6535.
- Ok KM, Chi EO (2006) Bulk characterization methods for non-centrosymmetric materials: second-harmonic generation, piezoelectricity, pyroelectricity and ferroelectricity. ChemSoc Rev 35: 710-717.
- Wu H, Pan S, Poeppelmeier KR (2013) Designing a Deep-Ultraviolet Nonlinear Optical Material with a Large Second Harmonic Generation Response. J Am ChemSoc 135: 4215-4218.
- Liang J, Li J,Xu R (2006) Preparation, Adsorption Properties, and Catalytic Activity of 3D Porous Metal–Organic Frameworks Composed of Cubic Building Blocks and Alkali-Metal Ions. AngewChemInt 45: 2546-2548.
- ZhaoSG, Luo JH (2014) Deep-Ultraviolet Transparent Phosphates RbBa2(PO3)5 and Rb2Ba3(P2O7)2 Show Nonlinear Optical Activity from Condensation of [PO4]3– Units. J Am ChemSoc 136: 8560-8563.
- Li LM, Zhang J (2013) Ionothermal Synthesis of Chiral Metal Phosphite Open Frameworks with In Situ Generated Organic Templates. InorgChem 52: 5654−5656.
- Wang G, Huang BB (2013) Cu2(OH)PO4, a Near-Infrared Activated Photocatalyst. AngewChemInt 52: 4810-4813.
- Wang XJ, Mao JG (2013) Syntheses, crystal structures of a series of novel alkali metal or alkaline earth metal phosphites. CrystEngComm 15: 2519-2526.
- Huang HL, Wang SL (2012) Intrinsic Optical Properties and Divergent Doping Effects of Manganese(II) on Luminescence for Tin(II) Phosphite Grown from a Deep-Eutectic Solvent. InorgChem 51: 1986-1988.
- Asl HY, Choudhury A (2015)Li3Fe2(HPO3)3Cl: an electroactive iron phosphite as a new polyanionic cathode material for Li-ion battery. J Mater Chem A 3: 7488-7497.
- Chung U, Rojo T (2011) Li1.43[FeII4.43FeIII0.57(HPO3)6]•1.5H2O: A PhosphiteOxoanion-Based Compound with Lithium Exchange Capability and Spin-Glass Magnetic Behavior. Chem Mater 23: 4317-4330.
- Maalej W, Vilminot E, Kurmoo M (2010) Crystal structure and magnetic properties of Na2NiII(HPO3)2. J Solid State Chem 183: 2650-2655.
- Cheng CC, Wang SL (2010) Synthesis and structural characterization of two cobalt phosphites: 1-D (H3NC6H4NH3)Co(HPO3)2 and 2-D (NH4)2Co2(HPO3)3. J Solid State Chem 183: 304-309.
- Crystal Clear (1999) Version 1.3.5,Rigaku Corp., Woodlands, TX, USA.
- SheldrickGM (1998) Crystallographic Software Package. SHELXTL, Version 5.1,Bruker-AXS, Madison, WI, USA.
- Spek AL (2003)Single-crystal structure validation with the program PLATON.J ApplCrystallogr 36: 7-13.
- Brown ID, Altermatt D (1985) Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. ActaCrystallogr B 41:244-247.
- Brese NE,KeeffeOM (1991) Bond-valence parameters for solids. ActaCrystallogr B 47: 192-197.
- Song JL, Mao JG (2015) Synthesis, crystal structures and properties of lead phosphite compounds. J Solid State Chem 231: 198-203.
- Kong K, Mao JG (2012) Explorations of new selenites of the group IIIA and IVA metals. J Solid State Chem 190: 118-125.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences