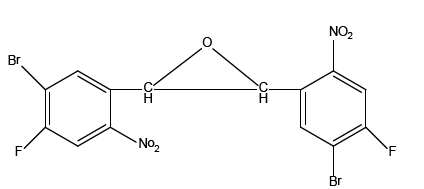
Crystal structure of 2, 3-Bis (5-bromo-4-fluoro-2- nitrophenyl) oxirane
T Sankar, Potharaju Raju, Arasambattu K Mohanakrishnan, S Naveen, N K Lokanath, K Gunasekaran
T Sankar1, Potharaju Raju2, Arasambattu K Mohanakrishnan2, S Naveen3, N K okanath4, K Gunasekaran1*
1Centre of Advanced Study in Crystallography and Biophysics, University of Madras, Guindy Campus, Chennai, India
2Department of Organic Chemistry, University of Madras, Guindy Campus, Chennai, India
3Institution of Excellence, University of Mysore, Manasagangotri, Mysore, India
4Department of Studies in Physics, University of Mysore, Manasagangotri, Mysore, India
- *Corresponding Author:
- K. Gunasekaran
Centre of Advanced Study in Crystallography and Biophysics, University of Madras, Guindy Campus, Chennai, India
Tel: +044-2220 2771
E-mail: gunaunom@gmail.com
Abstract
Single crystals of 2, 3-Bis (5-bromo-4-fluoro-2-nitrophenyl) oxirane (BrFOXI) were grown by slow evaporation method at room temperature. Crystallographic data set was collected using ref. [1] Smart Apexx II x-ray diffraction. Single crystal x-ray diffraction analysis reveals that crystallizes in the monoclinic, space group P2 (1)/c with unit cell dimensions, a=14.3073(19) Å, b=7.0042(9) Å, c=15.682(2) Å and β=106.664(4)°. The crystal structure was controlled by C-H…O types of intra and intermolecular interaction.
https://marsbahislinki.com https://marsbetgiris.com https://mobilebahiss.com https://1xbetsgirisi.com https://onwinegiris.com https://betistegiris.com https://piacasinogiris.com https://holiganbahiss.com https://holiganbetting.com https://girisholigan.com https://mostbetegiris.com https://mariobetoyna.com https://meritgirisi.com https://meritkinge.com https://kingmerite.com https://nanogiris.com https://casinoplusa.com https://casinoplusgiris.com https://betriyal.net https://pluscasinogiris.com
Keywords
Epoxy; Molecular structure; Crystal packing; Dimer formation
Introduction
Epoxy compounds are found to be useful in paint manufacturing, composite formations, development of adhesives as well as in many microelectronic applications such epoxy molding, bio medical applications [2-4]. Epoxides are organic three-membered compounds, where an oxygen atom is attached to two adjacent carbon atoms arise from oxidative metabolism of endogenous, as well as xenobiotic compounds. The resultant epoxides are typically unstable in aqueous environments and chemically reactive. The xenobiotics and certain endogenous substances which include epoxide intermediates have been implicated in genetic mutations and as cancer causing agents [5,6]. It is of vital importance for us to study the structure of epoxide compounds to understand the relationship in the above mentioned biological process. In view of the above said importance the properties of epoxides, the crystal structural studies of the compound mentioned is carried out.
Experimental
Synthesis of BrFOXI
The reaction of 5-bromo-4-fluoro-2-nitrobenzaldehyde (0.5 g, 2.01 mmol) with triethyl c(0.67 g, 4.03 mmol) in the presence of ZnBr2 (0.05 g, 0.20 mmol) at room temperature for 10 min followed by different procedures using the above using mentioned general procedure afforded trans-epoxide as a colorless solid. Single crystals suitable for X-ray diffraction studies were obtained by slow evaporation of the compound in chloroform/ ethyl acetate.
Data Collection
X-ray diffraction intensity data were collected for all three compounds on Bruker Kappa Apex II single crystal X-ray diffractometer equipped with graphite mono- chromate CuKα (λ=1.54178 Å) radiation and CCD detector. Crystals were cut to suitable size and mounted on a glass fiber using cyano acrylate adhesive. The unit cell parameters were determined from 36 frames measured (0.5° phi-scan) from three different crystallographic zones and using the method of difference vectors. The intensity data were collected with an average fourfold redundancy per reflection and optimum resolution (0.75 Å). The intensity data collection, frames integration, Lorentz and polarization correction and decay correction were done using SAINT-NT (version7.06a) software. Empirical absorption correction (multi-scan) was performed using SADABS program.
Results and Discussion
Structure Solving and Refinement
Crystal structure was solved by Direct Methods using SHELXS-97. All the non-hydrogen atoms were located without any difficulty. The structure was then refined by full-matrix least-squares method using SHELXL- 97. They arrived model was refined using isotropic thermal parameters followed by anisotropic thermal parameters refinements. After completion of the refinement where R factor is converged with negligible shift/e.s.d and agreeable Goof and other parameters, hydrogen atoms were positioned geometrically C—H=0.93–0.98 Å and allowed to ride on their parent atoms, with Uiso(H) =1.5Ueq(C) for methyl H 1.2Ueq(C) for other H atoms. The relevant crystallographic detail is given in (Table 1).
| Parameters | Values |
|---|---|
| Empirical formula | C14H6Br2F2N2O5 |
| Formula weight | 480.03 |
| Temperature | 296(2) K |
| Wavelength | 1.54178 Å |
| Crystal system, space group | monoclinic, P2(1)/c |
| Unit cell dimensions | a=14.3073(19) Å |
| b=7.0042(9) Å, β=106.664(4)° | |
| c=15.682(2) Å | |
| Volume | 1505.5(3)Å3 |
| Z, Calculated density | 4, 2.118 Mg/m3 |
| Absorption coefficient | 7.366 mm-1 |
| F(000) | 928 |
| Crystal size | 0.29 × 0.28 × 0.27 mm |
| Theta range for data collection | 3.22 to 64.93° |
| Limiting indices | -16<=h<=13, -8<=k<=8, -16<=l<=18 |
| Reflections collected/unique | 8652/2463 [R(int)=0.0541] |
| Completeness to theta=64.93 | 95.90% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2463 / 0 / 226 |
| Goodness-of-fit on F^2 | 1.616 |
| Final R indices [I>2sigma(I)] | R1=0.0628, wR2=0.1856 |
| R indices (all data) | R1=0.0636, wR2=0.1874 |
| Largest diff. peak and hole | 1.416 and -1.088 e. Å-3 |
Table 1: Crystal data for BrFOXY.
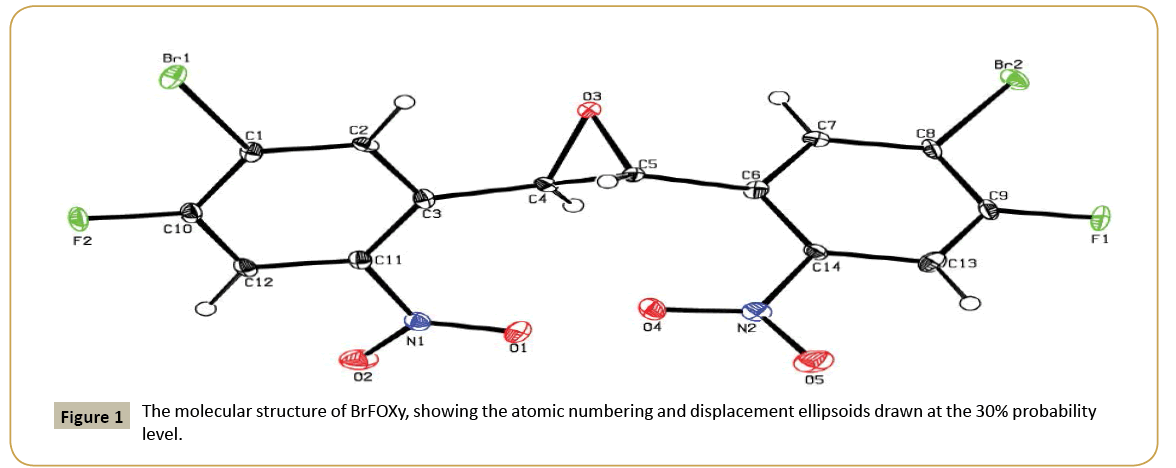
The molecular structure (ORTEP diagram) of BrFOXY is shown in (Figure 1). The bond lengths and bond angles are listed in (Table 2). The epoxy ring (O3/C4/C5), its looks like a triangle shape it form planar conformation and it is oriented axially with two halogen substituted phenyl rings (C1/C2C3/C10/C11/C12/Br1/ F2) and (C6/C7/C8/C9/C13/C14/Br2/F1) makes dihedral angles of 64.5(3)° and 66.5(3)°, respectively. The dihedral angle between the two halogen substituted phenyl rings is 60.5(1)°.
| Atoms | x | y | z | U(eq) |
|---|---|---|---|---|
| Br1 | 6565(1) | 3928(1) | 4916(1) | 22(1) |
| Br2 | -951(1) | 1468(1) | 3747(1) | 21(1) |
| F1 | -1559(2) | 1236(4) | 1743(2) | 24(1) |
| F2 | 7214(2) | 194(4) | 4300(2) | 23(1) |
| O1 | 3289(2) | -2510(6) | 2592(2) | 29(1) |
| O2 | 4534(3) | -4355(6) | 3116(2) | 28(1) |
| O3 | 2827(2) | 2083(5) | 4090(2) | 19(1) |
| O4 | 2441(2) | 1337(5) | 1470(2) | 24(1) |
| O5 | 1110(3) | 1433(6) | 375(3) | 32(1) |
| N1 | 4121(3) | -2798(6) | 3044(3) | 18(1) |
| N2 | 1548(3) | 1434(6) | 1174(3) | 18(1) |
| C1 | 5787(3) | 1867(7) | 4333(3) | 15(1) |
| C2 | 4791(3) | 1909(7) | 4170(3) | 14(1) |
| C3 | 4212(3) | 403(7) | 3747(3) | 14(1) |
| C4 | 3145(3) | 487(7) | 3645(3) | 15(1) |
| C5 | 2516(3) | 2043(7) | 3129(3) | 14(1) |
| C6 | 1441(3) | 1745(6) | 2738(3) | 14(1) |
| C7 | 859(3) | 1704(6) | 3307(3) | 14(1) |
| C8 | -150(3) | 1512(6) | 2974(3) | 16(1) |
| C9 | -584(3) | 1382(7) | 2061(4) | 19(1) |
| C10 | 6236(3) | 254(7) | 4115(3) | 17(1) |
| C11 | 4695(3) | -1158(6) | 3520(3) | 15(1) |
| C12 | 5688(4) | -1269(7) | 3703(3) | 16(1) |
| C13 | -37(4) | 1379(7) | 1475(3) | 19(1) |
| C14 | 976(3) | 1540(7) | 1826(3) | 15(1) |
Table 2: Atomic coordinates (× 104) and equivalent isotropic displacement
parameters (Å2 × 103) for the non-hydrogen atoms of BrFOXY.
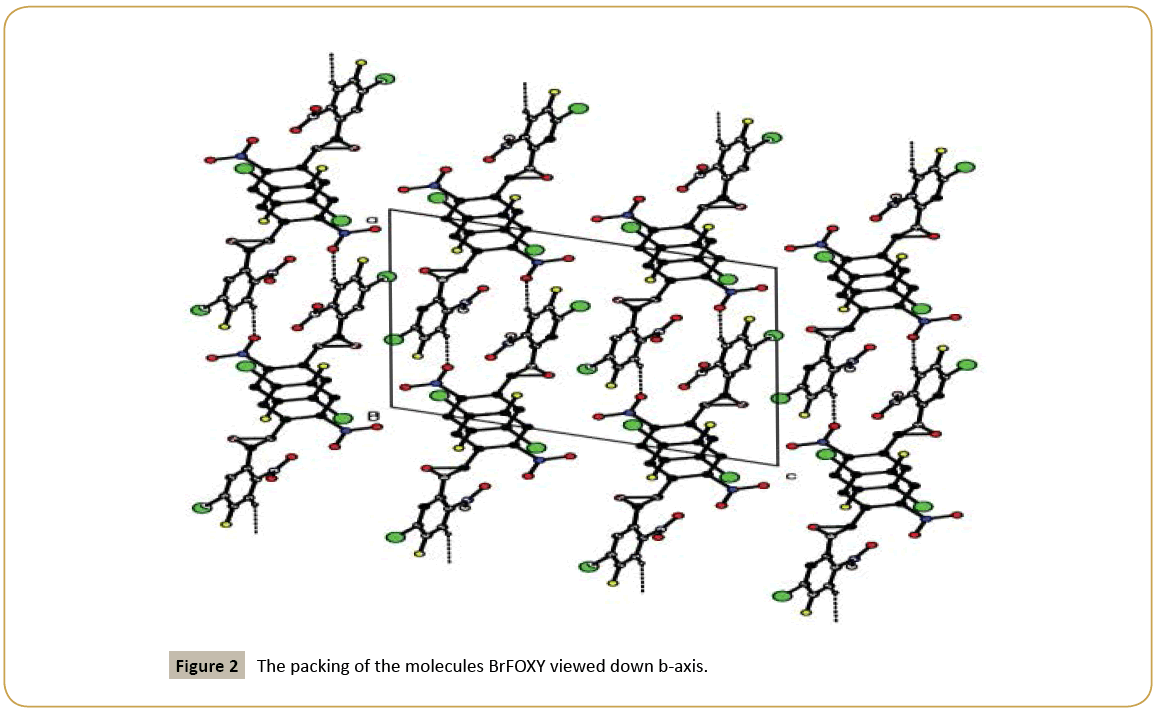
The nitro groups (N1/O1/O2) and (N2/O4/O5) are equatorially oriented with the halogen substituted phenyl ring (C1/C2C3/ C10/C11/C12/Br1/F2) and (C6/C7/C8/C9/C13/C14/Br2/F1) which are evidenced by the torsion angle values are [O1/N1/C11/ C12=] -153.3(4)°; [O2/N1/C11/C3=] -156.1(4)°; [O4/N2/C14/C6=] 7.2(7)° and [O4/N2/C14/C13=] -171.4(4)°, respectively. C-H…O types of intermolecular interaction makes C(10) chain running along a-direction (Figure 2). Relevant hydrogen bond details are given in (Table 3).
| D-H…A | D-H | H…A | D…A | DHA |
|---|---|---|---|---|
| C12-H1…O4i | 0.93 | 2.44 | 3.235(7) | 143 |
Table 3: Hydrogen bond interactions for BrFOXY [Å and ≡]. Symmetry code: 1-x,-1/2+y, 1/2-z.
Acknowledgements
The authors are thankful to Institution of Excellence, University of Mysore for providing the single-crystal X-ray diffraction facility.
Additional Information
Crystallographic data for the structures reported here have been deposited with CCDC Deposition No’s CCDC 1408773, 1408774 and 1408775. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, Fax: (+44) 1223 336 033; E-mail: deposit@ccdc.cam.ac.uk
References
- Bruker (2008) APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Kim WG, Lee JY (2002) Curing characteristics of epoxy resin systems that include a biphenyl moiety. J Appl Polym Sci 86: 1942-1952.
- Lee H and Neville K (1990) In Handbook of Epoxy Resins. New York: McGraw-Hill, USA.
- Adrian JF, Curtis J, Omiecinski (2000) Epoxide hydrolases: biochemistry and molecular biology. Chemico-Biological Interactions 129: 41-59.
- Guengrich (1982) Epoxide Hydrolase: Properties and Metabolic roles. Rev Bioch em Toxicol 400: 569-588.
- Sayer (1985) Stereoselectivity of Microsomal Epoxide Hydrolase toward Diol Epoxides and Tetrahydroepoxides Derived from Benz[u]anthrac. J Biol Chem 260: 1630-1640.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences