Bis(1-(2,6-Xylyl)-2- Imidazolidinethione Gold(I) and Copper(I) Halides: Synthesis, Characterization And Structure
Brandon Quillian, John W Haddock, Clifford W Padgett, Jessica L Gray, Hans-Jorg Schanz
Brandon Quillian1*, John W Haddock1, Clifford W Padgett1, Jessica L Gray2, Hans-Jörg Schanz2
1Department of Chemistry and Physics, Armstrong State University, 11935 Abercorn Street, Savannah, GA, USA
2Department of Chemistry, Georgia Southern University, 532 College of Education Dr., Statesboro, GA, USA
- *Corresponding Author:
- Prof. Brandon Quillian
Department of Chemistry and Physics, Armstrong State University, 11935 Abercorn Street, Savannah, GA, 31419 USA
Tel: 1.912.344.2977
Fax: 1.912.344.3432
E.mail: brandon.quillian@armstrong.edu
Abstract
In an effort to better understand the steric impact of sterically bulky heterocyclic thioureas on group 11 transition metals, we began a study with 1-(2,6-xylyl)-2-imidazolidinethione. Reaction of 1-(2,6-xylyl)-2- imidazolidinethione (L) with chloroauric acid (HAuCl4) affords the cationic complex, [bis(1-(2,6-xylyl)-2-imidazolidinethione)gold(I)] chloride, [L2Au(I)] Cl [1], while reaction of L with either copper (II) bromide or chloride (CuBr2 and CuCl2) under similar conditions yields isostructural, trigonal planar, bis(1-(2,6-xylyl)-2-imidazolidinethione copper(I) halide complexes, (L2CuX; X=Br=2; X=Cl=3). Complex 1 crystallizes in the orthorhombic unit cell (Pbcn space group) and the crystal structure displays an essentially linear, twocoordinate gold atom [S(1)−Au−S(1?) bond angle=171.79(7)°] with the N−H groups of the ligand participating in intermolecular hydrogen bonds with the chloride anion. Compounds 2 and 3 are both isostructural and isomorphic, crystallizing in the primitive monoclinic cell in the P21/c space group with nearly identical unit cell dimensions. The crystal structures of 2 and 3 display a trigonal planar three coordinate copper atom with the ligands held in a “syn-periplanar” type conformation with respect to the C(N-aryl)−S...S−C(N-aryl) dihedral plane (13.149(9)? for 2 and 17.45(19)? for 3). Both N−H groups participate in intramolecular hydrogen bonding with each respective halogen in 2 and 3. All complexes were characterized by 1H and 13C NMR spectroscopy and IR spectroscopy, elemental analysis and single crystal X-ray crystallography.
Keywords
1-(2,6-Xylyl)-2-Imidazolidinethione; Gold; Copper; X-Ray Structure; Intramolecular hydrogen bonds; Intermolecular Hydrogen bonds; Cationic; C−S bond; Au−S bond; Cu−S bond
Introduction
Heterocyclic thioureas have been the subject of exploration for several sectors of chemistry. Arguably, their most significant impact has been in the development of carbene chemistry, wherein reduction of thioureas with potassium metal affords a number of N-heterocyclic carbene ligands [1]. Heterocyclic thiourea ligands are ambidentate, monodentate donors, which can display thionethiol tautomerism via electron delocalization of the thioamide functional group. Despite their multiple bonding modes (η1/μ2- thione or σ-thiolate or κ2-N,S bonding) [2], the electron density of the S=C(N2) HOMO largely resides on the sulfur atom, resulting in primarily sulfur coordination to metals [3]. However, harder metals such zinc (II) have shown some preference for η1–N coordination [4]. Given that heterocyclic thiourea ligands were once avoided in transition metal-catalyzed applications and even considered a catalyst poison, it is notable that they have recently been used to prepare remarkably stable palladium complexes for applications in catalytic Heck cross coupling reactions [5,6]. Heterocyclic thiourea ligands have also contributed to the development of group 11 transition metal coordination [2], medicinal [7,8] and reaction chemistry. Our recent investigations into the bonding and properties of methimazole on gold led to the discovery of a cationic tetrakis(methimazole)gold(III) complex [9]. The coordination chemistry of heterocyclic thiourea ligands on the group 11 elements have largely been focused on those possessing small N-alkyl substituents (methyl, propyl, isopropyl, ethyl) [4,10-13]. It was surprising that the rich and developed group 11–heterocyclic thione chemistry lacked sterically bulky N-substituents such as substituted phenyl groups. In an effort to better understand the steric impact of bulky heterocyclic thioureas on group 11 transition metals, we began a study of the coordination chemistry of 1-(2,6-xylyl)-2-imidazolidinethione on copper and gold. Herein, we discuss the bonding, structure and spectroscopy of bis(1-(2,6-xylyl)-2-imidazolidinethione)gold(I) and copper(I) compounds. Notably, these compounds represent some of the most sterically encumbered heterocyclic thiourea gold and copper compounds currently known.
Results and Discussion
1-(2,6-xylyl)-2-imidazolidinethione (L) was prepared in a twostep synthesis in 63% overall yield, applying modified literature procedures for N-aryl-1,2-ethanediamines and their conversion with carbon disulfide (Equation 1) [14-16]. Ligand (L) has been described before and characterized by its elemental CHN analysis data. We report herein its full characterization (see Experimental Section).
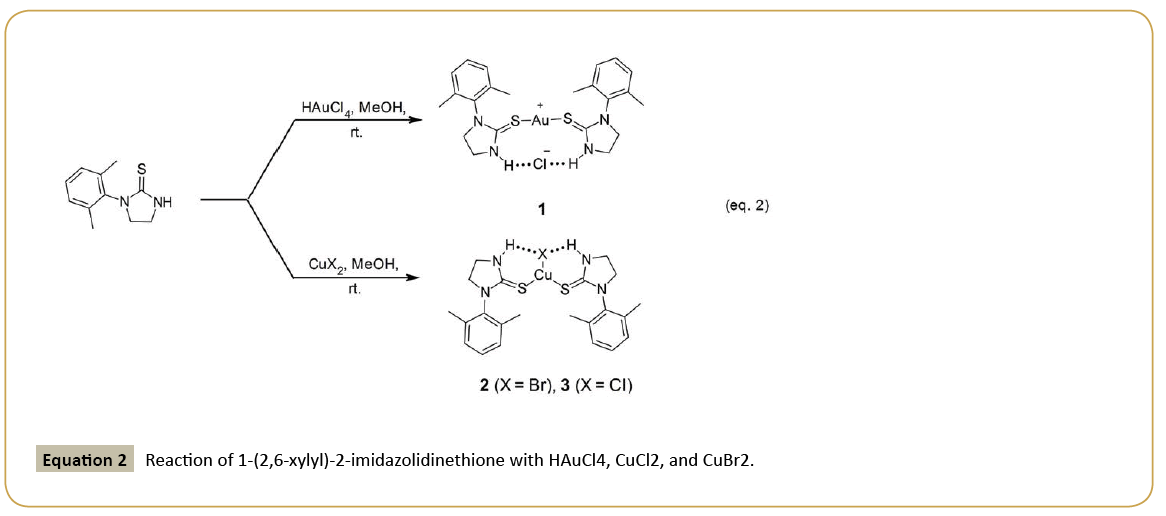
Reaction of 1-(2,6-xylyl)-2-imidazolidinethione (L) with chloroauric acid (HAuCl4) in methanol affords the cationic complex, [bis(1- (2,6-xylyl)-2-imidazolidinethione) gold(I)] chloride, [L2Au(I)]Cl [1], while reaction of L with either copper(II) bromide or chloride (CuBr2 and CuCl2) under similar conditions yields isostructural, trigonal planar, bis(1-(2,6-xylyl)-2-imidazolidinethione copper(I) halide complexes, (L2CuX; X=Br=2; X=Cl=3) (Equation 2). In either case, the metal is reduced in oxidation state from its halogen precursor.
The diastereotopic CH2 groups of the heterocyclic ring in 1 appear as two sets of second-order ddd (2JH-H=1, 3J(cis)H-H=2 Hz, 3J(trans) H-H=7 Hz) at 4.02 ppm and 3.88 ppm in its 1H NMR spectrum. A characteristic low field resonance between 8-10 ppm attributed to an N−H group was not observed in the 1H NMR spectrum due to possible proton-deuterium exchange with CD3OD caused by the acidity of the N–H group (5-11 pKa) [4]. Moreover, the N–H stretch in the infrared spectrum appears as a broad, ill-defined stretch (2750-3250 cm-1) and is partially obscured by the aliphatic C–H stretches. The lack of an N–H resonance in the 1H NMR and the obscured N–H infrared stretch of 1 suggests that the ligands are bound to the metal via a thiolate bonding mode and implying S–Au σ-bonds. In contrast, we suggest, based on previously reported Au–S and S=C bond distances for similar compounds (vide infra), that the ligands in 1 are bound to gold as a thione with mere S–Au η-bonds.
The 1H NMR resonances of the copper complexes 2 and 3 are broader and ill-defined when compared to those of complex 1. For example, in 2 the diastereotopic CH2 groups appear as a single broad multiplet at 3.93 ppm; however, in 3 two broad multiplets are observed for the CH2 groups [4.12-4.44 ppm, 3.89-4.11 ppm] and the aromatic resonances coalesce into a large broad singlet at 7.13 ppm (see Supporting Information). In both cases, an obvious broad NH resonance is observed far down field (2=8.64 ppm; 3=8.70 ppm). In addition, the infrared spectra of 2 and 3 clearly show the characteristic N–H stretch (2=3234 cm-1, 3=3205 cm-1). These observations are likely a consequence of the more acute bond angles around the Cu atom and hydrogen bonding interactions between the H−N unit of the ligand and respective halide (vide infra), which hinder free rotation of the ligand along the S–Cu bond at a rate slower than the NMR timescale.
Single crystal X-ray analysis reveals that complex 1 crystallizes in the orthorhombic unit cell (Pbcn space group) with an essentially linear coordination sphere around the two-coordinate gold atom [S(1)−Au−S(1') bond angle=171.79(7)°] (Figure 1). The heterocyclic thiourea ligands are crystallographically equivalent, thus the asymmetric unit represents only one-half of the molecule [Symmetry operation=-X+1, Y, -Z+1/2+1]. Unlike carbonyls, which have distinctive C=O stretches in the infrared spectrum (1650– 1850 cm-1), it is more difficult to distinguish a metal coordinated S–C stretch from an S=C stretch by infrared spectroscopy due to their nearly identical stretching frequencies (500-600 cm-1) and complexity of the fingerprint region. While several articles have reported thione M−S=C (M=Metal) stretches in the 500-600 cm-1 range [17,18], it is customary to observe the C=S stretch of the free ligand in the region of 1100-1160 cm-1 [19]. In an effort to determine a correlation between bond distance and bond order, a collection of S−Au, S−C, and S=C bond distances reported for thione and thiolate gold complexes were compiled [Table 1 (thione) and 2 (thiolate)]. We have also included the structures of the ligands associated with each complex in Figure 2. The S−Au bond distances for purported thione−gold complexes range from 2.25-2.357 Å (2.299 Å avg.), whereas the S−Au bond distances for purported thiolate−gold complexes varies from 2.279−2.409 Å with an average bond distance of 2.334 Å. Surprisingly, the S− Au σ-bond distances of gold-thiolate complexes are statistically longer than their dative-bound thione congeners. This suggests the metal-to-sulfur π-back bonding is more prevalent in the thione-gold complexes, attributed to the presence of the C=S π-bond. Indeed, the Au(1)−S(1) bond length in 1 (2.2952(15) Å) most closely aligns with those of gold−thione complexes. However, we recently reported a tetrakis-(methimazole) gold(I) complex with substantially longer Au−S bond distances (2.346(3) Å and 2.357(3) Å) [9]. Data from Tables 1 and 2 show, unsurprisingly, that the range of S=C bond distances [1.67-1.728 (1.716 avg.)] of gold-thione complexes are shorter than the S−C bond distances of gold-thiolate complexes [1.709-1.770 (1.730 avg.)]. Again, the S=C bond in 1 fits well with other reported gold−thione complexes. The chloride anion in 1 is separated from the gold cation by 3.295(3) Å. The bulkiness of the xylyl ligand in 1 precludes a nearly anti-periplanar C−S· · ·S−C dihedral angle (108.8 (5)°) as reported for the bis(imidazolidine-2-thione) and bis(methyl-imidazolidine-2-thione) gold(I) complexes (-174.0(2)° and -167.5(1)°, respectively) [20]. Instead, the two heterocyclic ring planes assume a dihedral angle of -78.6(4)° with respect to each other and each is nearly orthogonal to their aryl ring plane (85.7 (2)°). These manifestations are most likely influenced by the intermolecular hydrogen bonding interaction between the N−H donor and chloride anion acceptor (3.189(4) Å). Units of 1 pack centrosymmetrically into the unit cell with the gold atoms sitting on the edges of the b-c plane (Figure 3), and there are no close “aurophilic” Au−Au bond distances (Au−Au metallic bond distance=2.884 Å) [21].
| Entry | Formula | Au––S (Å) | S–C (Å) | Reference |
|---|---|---|---|---|
| 1 | [(L1)2Au]+ | 2.2927(15) 2.2962(15) | 1.714(6) 1.725(6) |
[20] |
| 2 | [(L2)2Au]+ | 2.2976(12) 2.2985(13) |
1.721(5) 1.720(4) |
[20] |
| 3 | [(L3)2Au]+ | 2.2856(10) 2.2904(10) | 1.722(4) 1.720(4) |
[20] |
| 4 | (L4)AuCl | 2.25(1) | 1.67(4) | [22] |
| 5 | (L2)2AuCl·H2O | 2.279(8) | 1.718(22) | [23] |
| 6 | [(L5)4Au]+3 | 2.346(3) 2.357(3) |
1.728(7) 1.724(7) |
[9] |
Table 1: Au–S and S=C bond lengths of previously reported Au–thione complexes.
| Entry | Formula | Au––S (Å) | S–C (Å) | Reference |
|---|---|---|---|---|
| 7 | [(L6)4Au]- | 2.352(1) 2.356(1) |
1.736(3) | [24] |
| 8 | (L6)Au(PPh3) | 2.352(1) | 1.740(4) | [25] |
| 9 | (L7)Au(PPh3) | 2.304(2) | 1.733(7) | [25] |
| 10 | [(L7)4Au]- | 2.356(1) 2.358(1) |
1.743(6) 1.735(6) |
[25] |
| 11 | [(L7)2Au]- | 2.279(2) 2.288(2) |
1.748(7) 1.725(7) |
[25] |
| 12 | [Au(Hdamp-C1)(Cl)(L8)2]+2 | 2.326(3) 2.311(3) |
1.709(5) 1.714(5) |
[26] |
| 13 | [Au(Hdamp-C1)(Cl)(L9)2]+2 | 2.337(3) 2.334(3) |
1.735(12) 1.741(12) |
[26] |
| 14 | Au(Hdamp-C1)(L10)3 | 2.318(2) 2.329(2) 2.409(2) |
1.723(7) 1.732(7) 1.728(7) |
[26] |
| 15 | [Au(L11)4]- | 2.340(1) | 1.770(6) | [27] |
Table 2: S-Au and S-C bond lengths of previously reported Au–thiolate complexes. Note: damp = 2-(dimethylaminomethyl)phenyl-C1,N.
The single crystal X-ray structures of 2 and 3 are both isostructural and isomorphic. Both crystallize in the primitive monoclinic cell in the P21/c space group with nearly identical unit cell dimensions (See Table 3). The structure of 2 is a representative example for both (Figure 4). The structure of 2 displays a central copper atom in pseudo-trigonal planar geometry. Interestingly, the plane of the imidazolidine ring is nearly coplanar with the CuBrS2 plane and the N-Aryl groups are aligned in a “syn-periplanar” geometry with respect to the C(1)−S · · · S−C(12) dihedral plane (13.149 (9)°), while the N-aryl ring plane is essentially orthogonal to the plane of the imidazolidine ring (dihedral angle=89.14 (3)°), as observed in 1. The Br(1)–Cu(1)–S(1) and Br(1)–Cu(1)–S(2) bond angles (121.05(5)° and 123.21(6)°, respectively) are slightly more obtuse than the S(1)–Cu(1)–S(2) bond angle (115.70(2)°), which is likely the result of the hydrogen bonding interaction between the H–N donor and Br acceptor (N(2)−Br(1)=3.4103[18] Å; N(4)− Br(1)=3.4359[17] Å). The heterocyclic thione ligand bearing the S(2) atom has its thionyl carbon (C=S) bent out of the S2CuCl plane (dihedral angle: Br(1)–Cu(1)–S(2)–C(12)=7.857(3)°) considerably more as compared to that bearing the S(1) atom (dihedral angle: Br(1)–Cu(1)–S(1)–C(1)=1.0259(10)°. Moreover, the Cu(1)–S(1) bond length (2.2179(12) Å) is slightly shorter than the Cu(1)–S(2) bond distance (2.2361(13) Å); however, both are similar to those reported for other heterocyclic thione Cu(II) complexes [13]. The widened Br(1)–Cu(1)–S(2) bond angle (123.21(6)°) and longer Cu(1)–S(2) bond length is likely the result of packing effects (vide infra). These structural manifestations are present in 3 as well (vide infra). The Br(1)–Cu(1) bond distance (2.3881(15) Å) is within the typical range for Cu(I)-Br distances [11].
| Chemical formula | C22H26AuClN4S2 (1)1 | C22H28BrCuN4S2 (2)2 | C22H28ClCuN4S2 (3)2 |
|---|---|---|---|
| Mr | 643.03 | 556.06 | 511.59 |
| Crystal system, space group | Orthorhombic, Pbcn | Monoclinic, P21/c | Monoclinic, P21/c |
| Temperature (K) | 173 | 173 | 173 |
| a, b, c(Å); ß(°) |
11.830 (4), 13.973 (6), 14.462 (6) | 17.236 (12), 10.366 (7), 14.720 (11); ß(°)108.102(7) | 17.122 (9), 10.337 (5), 14.458 (8); ß(°) 107.614(5) |
| V(Å3) | 2390.6 (17) | 2500 (3) | 2439 (2) |
| Z | 4 | 4 | 4 |
| Radiation type | Mo Ka | Mo Ka | Mo Ka |
| µ (mm-1) | 6.48 | 2.66 | 1.19 |
| Crystal size (mm) | 0.54 × 0.45 × 0.23 | 0.22 × 0.22 × 0.12 | 0.70 × 0.40 × 0.40 |
| Data Collection | |||
| Diffractometer | Rigaku XtaLAB mini diffractometer |
Rigaku XtaLAB mini diffractometer |
Rigaku XtaLAB mini diffractometer |
| Absorption correction | Multi-scanREQAB (Rigaku, 1998) | Multi-scanREQAB (Rigaku, 1998) | REQAB (Rigaku, 1998) |
| Tmin, Tmax | 0.115, 0.225 | 0.459, 0.727 | 0.373, 0.621 |
| No. of measured, independent and observed [F2> 2.0s(F2)] reflections | 23558, 2744, 2375 | 25942, 5702, 3772 | 25264, 5553, 4516 |
| Rint | 0.075 | 0.116 | 0.091 |
| (sin θ/λ)max (Å-1) | 0.65 | 0.649 | 0.649 |
| Refinement | |||
| R[F2> 2s(F2)], wR(F2), S | 0.041, 0.113, 1.11 | 0.050, 0.140, 1.07 | 0.041, 0.103, 1.04 |
| No. of reflections | 2748 | 5702 | 5553 |
| No. of parameters | 137 | 275 | 275 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å-3) | 2.10, -2.50 | 0.73, -0.70 | 0.43, -0.75 |
1Computer programs: CrystalClear-SM Expert 2.0 r15 (Rigaku 2011), CrystalClear-SM Expert 2.0 r15, SIR92 (Altomare et al.), SHELXL97 (Sheldrick 2008), Crystal Structure 4.0 (Rigaku 2010).
2Computer programs: CrystalClear-SM Expert 2.0 r15 (Rigaku 2011), ShelXT (Sheldrick 2015), SHELXL (Sheldrick 2008), Olex2 (Dolomanov et al.).
Table 3: Single Crystal X-ray Study Experimental Details for complexes 1-3.
The geometry and coordination features of 3 mirror that of 2 (see Supporting Information). The Cu(1)–Cl(1) bond distance of 3 is 2.2599(12) Å and within range of similar copper(1) thiourea complexes [28]. The Cu(1)–S(1) and Cu(1)–S(2) bond distances (2.2083(14) Å and 2.2267(13) Å) on average are shorter than those in 2. This is likely due to the smaller chloride atom, allowing for closer approach to the metal. Similar to that of 2, the structure of 3 reveals that as the Cl–Cu–S bond angle widens the Cu–S bond distance increases (Cu(1)–S(1)=2.2086(11) Å, Cl(1)–Cu(1)–S(1)=120.94(3)°; Cu(1)–S(2)=2.2262(12) Å, Cl(1)– Cu(1)–S(2)=122.94(4)°). We conducted computational studies on compound 3 optimized in Gaussian W09 using the B3LYP level of theory with the 6-31G* basis set to determine if the variances in the S−Cu bond distances and X−Cu−S bond angles in 2 an 3 were a result of electronic perturbations or simply a consequence of packing. Our computational study agrees with the single crystal X-ray study and suggests the asymmetry found in 3 is a result of packing effects, as the gas phase optimized structure of 3 (3') is clearly symmetrical (Figure 5) with identical Cu−S bond lengths (2.182 Å) and Cl–Cu–S bond angles (118.97°).
Conclusions and Summary
Reaction of 1-(2,6-xylyl)-2-imidazolidinethione with H[AuCl4], CuBr2 or CuCl2 resulted in the reduction of the transition metals and formation of the respective bis(1-(2,6-xylyl)-2- imidazolidinethione) Au(I) or Cu(I) halide complexes. While both group 11 metals in 1-3, Au and Cu, assume a +1 oxidation state, compound 1 exists as a cationic, two-coordinate Au complex, whereas the isostructural and isomorphic copper complexes 2 and 3 are neutral and three-coordinate with intact Cu−X bonds. The differences in coordination environments of 1 versus 2 and 3 are likely due to the softer Lewis acid properties of Au(I) as compared to Cu(I). The structures of all three complexes indicate hydrogen bonding between the N− H group of the thiourea ligand and halide. While the coordination mode of these complexes mimic those previously reported, the steric bulk of the ligand in the copper complexes appears to inhibit its free rotation along the Cu−S bond. These compounds are the first substituted N-Aryl heterocyclic thioureas coordinated to Au(I) and Cu(I) and adds some sterically encumber complexes to the well-developed group 11–heterocyclic thiourea coordination chemistry.
Experimental
All commercially available chemicals were purchased and used as received without further purifications. Bis(1-(2,6-xylyl)- 2-imidazolidinethione was prepared by literature methods [14-16]. Infrared spectra were recorded with a Perkin Elmer Spectrum-100 FTIR as thin-films on NaBr plates for liquids or ATR methods for solids, or a Thermo-Nicolet IS10 FTIR using ATR for liquids and solids. 1H NMR spectra were recorded on a JEOL JNMECP300 FT 300 MHz NMR or an Agilent 400 MHz NMR system. All 1H and 13C{1H} NMR spectra are referenced against residual proton signals (1H NMR) or the 13C resonances of the deuterated solvent (13C{1H} NMR) and chemical shifts are recorded in ppm (δ). Coupling constants (JHH values) are reported in Hz. Elemental analyses were performed at Atlantic Microlab, Inc., Norcross, GA. X-ray diffraction measurements were made on a Rigaku XtaLAB mini diffractometer.
Synthesis of N-(2’-aminoethyl)-2,6-dimethylaniline
In a modified literature procedure for N-aryl-1,2-ethanediamines [14,15], 2’-chloroethyldimethylamine hydrochloride (9.144 g, 78.83 mmol) was added to 2,6-dimethylaniline (25.308 g, 209.0 mmol) in a two-neck flask equipped with a thermometer. The solvent-free mixture was heated with stirring at 130°C for 3 h. The resulting dark reddish-brown solution solidified upon cooling to room temperature. Water (100 mL) was added and the mixture was stirred until a homogeneous biphasic solution formed. The solution was basified with sodium carbonate (4.186 g, 39.49 mmol) and extracted with CH2Cl2 (1 × 50 mL and 2 × 20 mL) to remove excess 2,6-dimethylaniline (15.763 g, 130.3 mmol) >95% purity (1H NMR spectroscopy). Sodium hydroxide (3.649 g, 91.23 mmol) was added to the aqueous phase and the mixture was extracted with CH2Cl2 (1 × 50 mL and 2 × 20 mL). The organic phases were combined, dried over anhydrous sodium sulfate and filtered to give the crude 2,6-dimethyl-N-(2’-aminoethyl)aniline (11.867 g) in >97% purity (1H NMR spectroscopy) as a viscous yellow liquid. For purification, the crude product was dissolved in ethanol (100 mL) and treated with concentrated aqueous hydrochloric acid (15 mL, approximately 12 M) in small portions over a period of 20 min. Toluene (20 mL) was added to induce precipitation and all solvent was removed under reduced pressure. Acetone (200 mL) was added to the residue and the resulting slurry was sonicated for 60 minutes. The slurry was filtered, washed with acetone (2 × 20 mL), and the solid was dried in the vacuum oven at 60°C for 4 h. The residue was dissolved in water (70 mL), sodium hydroxide was added (3.295 g, 82.38 mmol) and the mixture was extracted with CH2Cl2 (1 × 50 mL and 2 × 20 mL). The organic phases were combined, dried over anhydrous sodium sulfate and filtered to give the product (10.442 g, 63.57 mmol, 81%) in >99% purity (1H NMR spectroscopy) as a viscous light-yellow liquid. 1 H NMR (400 MHz, CDCl3, 24°C) δ: 7.00 (d, 2H, Ar-Hm, 3JH-H=7 Hz), 6.83 (t, 1H, Ar-Hp, 3JH-H=7 Hz), 3.04 (t, 2H, NH-CH2-CH2-NH2, 3JH-H=7 Hz), 2.91 (t, 2H, NH-CH2-CH2-NH2, 3JH-H=7 Hz), 2.32 (s, 6H, C6H3(CH3)2), 1.87 (br., 3H, NH-CH2CH2); 13C NMR (100.6 MHz, (CD3)2SO, 24°C) δ: 146.1 (Ar-Ci), 129.4 (Ar-Cp), 128.8 (Ar-Cm), 121.7 (Ar-Co), 50.9 (CH2NHAr), 42.5 (CH2NH2), 18.5 (C6H3(CH3)2). IR (ATR, neat) cm-1: 3366 (br., N-H str.).
Synthesis of 1-(2’,6’-dimethylphenyl)-2-imidazolidinethione
In a modified literature procedure [16], carbon disulfide (3.0 mL, 3.8 g, 50 mmol) was added in one portion to a solution of the aniline (6.401 g, 38.97 mmol) in 50 mL toluene. The mixture was heated to reflux for 2 h and then cooled to -20°C for 24 h. A crystalline solid precipitated. The slurry was filtered and the residue was washed with toluene (10 mL) and methyl t-butyl ether (2 × 10 mL). The residue then was dried in the vacuum oven at 60°C for 6 h to yield the product (6.286 g, 30.47 mmol, 78.2%) in >99% purity (1H NMR spectroscopy) as a light-yellow powdery solid. 1H NMR (400 MHz, (CD3)2SO, 24°C) δ: 8.31 (br, 1H, NH), 7.12 (m, 3H, C6H3), 3.83 (t, 2H, NH-CH2-CH2-NH2, 3JH-H=9 Hz), 3.66 (t, 2H, NH-CH2-CH2-NH2, 3JH-H =9 Hz,), 2.32 (s, 6H, C6H3(CH3)2); 13C NMR (100.6 MHz, (CD3)2SO, 24°C) δ: 181.8 (C=S), 137.1 (Ar-Ci), 136.6 (Ar-Co), 128.0 (Ar-Cm), 127.4 (Ar-Cp), 49.4 (CH2NAr), 41.9 (CH2NH), 17.3 (C6H3(CH3)2). IR (ATR, neat) cm-1: 3245 (br., N-H str.), 1289 (C=S str.).
Synthesis of [bis(1-(2,6-xylyl)-2-imidazolidinethione) gold(I)] chloride (1)
Bis(1-(2,6-xylyl)-2-imidazolidinethione (0.124 g, 0.61 mmol) and chloroauric acid trihydrate (HAuCl·3H2O) (0.100 g, 0.253 mmol) were dissolved in methanol in separate vessels (60 mL and 40 mL, respectively). The solutions were combined then sonicated until completely dissolved. An immediate color change from pale-yellow to orange was observed. The solution was allowed to evaporate slowly at room temperature to afford colorless X-ray quality crystals of the product. (0.065 g, 0.100 mmol, 40%) 1H NMR (300 MHz, CD3OD) δ: 7.20 (t, 1H, Arp-H, 3JH-H=7 Hz,), 7.10 (d, 2H, Arm-H, 3JH-H=7 Hz,), δ 4.02 (ddd, 2H, CH2, 2JH-H=1, 3J(cis)H-H=2 Hz, 3J(trans)H-H=7 Hz,), 3.88 (ddd, 2H, CH2, 2JH-H=1 Hz, 3J(cis)H-H=2 Hz, 3J(trans) H-H=7 Hz), δ 2.20 (s, 6H, CH3).13C NMR (75 MHz, CD3OD) δ: 178.5 (C=S), 138.2 (Ar-Ci), 136.6 (Ar-Co), 130.9 (Ar-Cp), 130.3 (Ar-Cmeta), 52.8 (CH2NH), 44.1 (CH2NAr), 18.0 (CH3). IR (ATR, neat) cm-1: 3062 (N-H br. Str.), S=C (1239 str.). EA Theo(Found) C: 41.03(41.23), H:4.23(4.39), N 8.70(8.63).
Synthesis of [bis(1-(2,6-xylyl)-2-imidazolidinethione) copper(I) bromide (2)
Bis(1-(2,6-xylyl)-2-imidazolidinethione (0.125 g, 0.61 mmol) and CuBr2 (0.052 g, 0.22 mmol) were dissolved separately in methanol (100 mL and 20 mL, respectively). The solutions were combined and stirred, causing an immediate color change from paleyellow to dark brown with concomitant precipitation. Needlelike crystals formed upon slow evaporation of the solution. The crystals were re-crystallized from chloroform by slow evaporation to yield large pale-yellow crystals of the product (0.071 g, 0.126 mmol, 57% yield). 1H NMR (300 MHz, CDCl3) δ: 8.62 (bs, 2H, NH), 7.17 (dd, 2H, Ar-Ho, 3JH-H=9 Hz, 3JH-H=6 Hz ), 7.09 (bd, 4H, Ar-Hm, 3JH-H =7 Hz) δ 3.96 (bm, 4H, CH2-CH2), δ 2.24 (s, 6H, CH3). 13C NMR (75 MHz, CDCl3) δ: 180.6 (C=S) 136.9 (Ar-Ci), 135.2 (Ar-Co), 128.9 (Ar-Cp), 128.6 (Ar-Cm), 50.5(CH2-NH), 42.8(CH2NAr), 17.8 (CH3). IR, ATR (neat) cm-1: 3234 (br., N-H, med.), 1263 (S=C, str.). EA Theo. (Found) C: 47.52(47.57), H:5.08(5.16), N: 10.08(9.84).
Bis(1-(2,6-xylyl)-2-imidazolidinethionecopper(I) chloride (3)
Bis(1-(2,6-xylyl)-2-imidazolidinethione (0.127 g, 0.61 mmol) and CuCl2 (0.31 g, 0.23 mmol) were dissolved in methanol separately (100 mL and 50 mL, respectively). The solutions were combined and stirred. All solvent was removed, the residue was dissolved in chloroform and the solution was left to slowly evaporate to afford large yellowish crystals of the product. (0.069 g, 0.135 mmol, 59%). 1H NMR (300 MHz, CD2Cl2) δ 8.67 (bs, 1H, NH) 7.02-7.24 (m, 3H, Ar-H), δ 4.12-4. 44 (m, 2H, CH2), 3.89-4.11 (m, 2H, CH2), 2.23 (bs, 6H, CH3). 13CNMR (75 MHz, CD2Cl2) δ 167.62(C=S), 138.72(Ar- Ci), 135.07(Ar-Co), 129.31(Ar-Cp), 128.21(Ar-Cm), 40.78(CH2NH), 29.73(CH2NAr), 18.14(Cmethyl-Ar). IR(ATR neat) cm-1: 3223(N-H), 1262 (S=C str.).
Crystal structure determination of Complexes 1-3
A crystal was placed on the tip of a Mitigen micromount and X-ray intensity data were measured at low temperature (173(2) K) with graphite monochromated Mo Kα radiation (λ=0.71075 A°). Preliminary sets of cell constants were calculated from reflections harvested from one set of 12 images with frame times of 30 s. A randomly oriented region of reciprocal space was surveyed to the extent of one sphere and to a resolution of 0.77 A°. Three major sections of frames were collected with 1.0 steps in ω at three different ? settings. The intensity data were corrected for absorption and decay. Final cell constants were calculated from the strong reflections of xyz centroids from the actual data collection after integration. The structure was solved and refined using the Crystal Structure software package using default SHELXL-97 [29] or Olex2. A direct-methods solution was calculated that provided most of the non-hydrogen atoms from the E-map. Full-matrix leastsquares/ difference Fourier cycles were performed that located the remaining non-hydrogen atoms. All non-hydrogen atoms were refined with anisotropic displacement parameters. Most of the hydrogen atoms were placed in ideal positions and refined as riding atoms with relative isotropic displacement. The final refinement cycles led to R1=X and wR2=X.
Supporting Information Available
Computational methods and representative NMR and IR data spectra are available. Crystallographic data for structures 1–3 are deposited in the Cambridge Crystallographic Data Centre: CCDC-1423133 (1), CCDC-1422020 (2), CCDC-1422011 (3). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_ request/cif or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, CB2 1EZ, UK, Fax: +44 1223 336033.
Acknowledgements
BQ would like to acknowledge the American Chemical Society Petroleum Research Fund for support or partial support of this research (PRF# 53848-UNI3). HJS would also like to acknowledge the SEED funding program at Georgia Southern for financial support. The authors would also like to thank Armstrong State University and Georgia Southern University for their support.
Conflict of Interest
The authors declare no competing financial interest.
References
- Kuhn N, Kratz T (1993) Synthesis of Imidazol-2-Ylidenes by Reduction of Imidazole-2(3h)-Thiones. Synthesis-Stuttgart 561-562.
- Blower PJ, Dilworth JR (1987) Thiolato-Complexes of the Transition-Metals. Coordination Chemistry Reviews 76: 121-185.
- Liu S, Lei Y, Yang Z, Lan Y (2014) Theoretical study of the electron-donating effects of thiourea ligands in catalysis. Journal of Molecular Structure 1074: 527-533.
- Raper ES (1996) Complexes of heterocyclic thionates1. Complexes of monodentate and chelating ligands.Coordination Chemistry Reviews 153: 199-255.
- Chen W, Li R, Wu Y, Ding LS, Chen YC (2006) Self-supported thiourea-palladium complexes: Highly air-stable and recyclable catalysts for the Suzuki reaction in neat water. Synthesis-Stuttgart 3058-3062.
- Yang D, Chen YC, Zhu NY (2004) Sterically bulky thioureas as air- and moisture-stable ligands for Pd-catalyzed Heck reactions of aryl halides. Organic Letters 6: 4635-4635.
- Tardito S, Bussolati O, Maffini M, Tegoni M, Giannetto M, et al. (2007) Thioamido coordination in a thioxo-1,2,4-triazole copper(II) complex enhances nonapoptotic programmed cell death associated with copper accumulation and oxidative stress in human cancer cells. Journal of Medicinal Chemistry 50: 1916-1924.
- Santini C, Pellei M, Gandin V, Porchia M, Tisato F, et al. (2014) Advances in Copper Complexes as Anticancer Agents. Chemical Reviews 114: 815-862.
- Lynch WE, Padgett CW, Quillian B, Haddock J (2015) A square-planar hydrated cationic tetrakis(methimazole)gold(III) complex. ActaCrystallographica Section C-Structural Chemistry 71: 298.
- Raper ES (1985) Complexes of Heterocyclic Thione Donors. Coordination Chemistry Reviews 61: 115-184.
- Raper ES (1994) Copper-Complexes of Heterocyclic Thioamides and Related Ligands. Coordination Chemistry Reviews 129: 91-156.
- Raper ES (1997) Complexes of heterocyclic thionates. 2. complexes of bridging ligands. Coordination Chemistry Reviews 165: 475-567.
- Akrivos PD (2001) Recent studies in the coordination chemistry of heterocyclic thiones and thionates. Coordination Chemistry Reviews 213: 181-210.
- Isabel P, Caterina MC, Lopez J, Salerno A (2004) Synthesis and study of 1-aryl-1H-4,5-dihydroimidazoles. Synthesis-Stuttgart 851-856.
- Bessel M, Rominger F, Straub BF (2010) Modular Trimethylene-Linked Bisimidazol(in)ium Salts. Synthesis-Stuttgart: 1459-1466.
- Sztanke K, Pasternak K, Sidor-Wojtowicz A, Truchlinska J, Jozwiak K (2006) Synthesis of imidazoline and imidazo[2,1-c][1,2,4]triazole aryl derivatives containing the methylthio group as possible antibacterial agents. Bioorganic & Medicinal Chemistry 14: 3635-3642.
- Isab AA, Hussain MS (1985) Synthesis, C-13 NMRand IR Spectroscopic Studies of Gold(I) Complexes of Imidazolidine-2-Thione and Its Derivatives. Polyhedron 4: 1683-1688.
- Hussain MS, Hossain ML, Alarfaj A (1990) Mixed-Ligand Complexes of Gold(I) and Silver(I) with Heterocyclic Thiones. Transition Metal Chemistry 15: 120-125.
- Lambert JB, Shurvell HF, Lightner DA, Cooks RG (1998) Organic Structural Spectroscopy. Prentice-Hall Inc., Upper Saddle River.
- Friedrichs S, Jones PG (2004) Secondary interactions in gold(I) complexes with thione ligands, 3. Three ionic dimesylamides [1,2]. Zeitschrift Fur Naturforschung Section B-a Journal of Chemical Sciences 59: 1429-1437.
- BatcheldDN, Simmons RO (1965) X-Ray Lattice Constants of Crystals by a Rotating-Camera Method - Al Ar Au Caf2 Cu Ge Ne Si. Journal of Applied Physics 36: 2864.
- Hussain MS, Isab AA (1985) Gold(I) Complexes of N-Alkyl Substituted Imidazolidine-2-Thiones - Synthesis, Spectroscopic Studies and X-Ray Structure. Journal of Coordination Chemistry 14: 17-26.
- Jones PG, Guy JJ, Sheldrick GM (1976) Bis(Ethylenethiourea)Gold(I) Chloride Hydrate. ActaCrystallographica Section B-Structural Science 32: 3321-3322.
- Lang ES, Dahmer M, Abram U (1999) Tetraphenylphosphoniumtetrakis(1-methyl-1,2,3,4-tetrazole-5-thiolato-S)-aurate(III) hemihydrate. ActaCrystallographica Section C-Crystal Structure Communications 55: 854-856.
- Noth H, Beck W, Burger K (1998) The molecular structure of some transition metal complexes with 1,2,3,4-tetrazole-5-thiolate anions. European Journal of Inorganic Chemistry: 93-99.
- Abram U, Mack J, Ortner K, Muller M (1998) Reactions of dichloro[2-(dimethylaminomethyl)phenyl-C-1,N]gold(III), [Au(damp-C-1,N)Cl-2], with heterocyclic thiols. Evidence for Au-N bond cleavage and protonation of the dimethylamino group. Journal of the Chemical Society-Dalton Transactions: 1011-1019.
- Watase S, Kitamura T, KanehisaN, Nakamoto M, Kai Y, et al. (2003) Tetra-n-butylammoniumtetrakis(pentafluorobenzenethiolato-kappa S)aurate(III). ActaCrystallographica Section C-Crystal Structure Communications 59: M162-M164.
- Balewski L, Saczewski F, Bednarski PJ, Gdaniec M, Borys E, et al. (2014) Structural Diversity of Copper(II) Complexes with N-(2-Pyridyl)Imidazolidin-2-Ones(Thiones) and Their in Vitro Antitumor Activity. Molecules 19: 17026-17051.
- Sheldrick GM SHELXTL-97, Version 5.12, Bruker AXS Madison,WI53719, USA.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences