A Simple Method of Diagnostic Pathology for Identification of Crystal Deposits in Metabolic and Crystal Induced Diseases
Miklós Bély and Ágnes Apáthy
Miklós Bély1* and Ágnes Apáthy2
1Department of Pathology, Policlinic of the Order of the Brothers of Saint John of God in Budapest, Hungary
2Department of Rheumatology – St.Margaret Clinic Budapest, Hungary
- *Corresponding Author:
- Miklós Bély
Department of Pathology, Policlinic of the Order of the Brothers of Saint John of God in Budapest, H- 1027 Budapest, Frankel L.17-19, Hungary.
Tel: 3614388491
E-mail: dr.bely.miklos@gmail.hu
Received Date: January 21, 2016; Accepted Date: February 10, 2016; Published: February 15, 2016
Abstract
Gout, chondrocalcinosis and apatite rheumatism are regarded as distinct clinical entities. Exact clinical and/or histological diagnosis is needed, because of different outcome and therapy of these diseases. Authors demonstrate a simple and effective histological technique for exact identification of distinct crystal deposits in surgical specimens. The simplest and most effective “staining” technique to demonstrate crystals is “not-staining”. One of the major works of histochemistry does not discuss this simple method. In case of clinically or histologically suspected metabolic or crystal induced disease the tissue specimens should be evaluated (examined) in sections stained by haematoxylin-eosin and in unstained sections as well. The probability of crystal positive cases is much higher in unstained sections viewed under polarized light in comparison with haematoxylin-eosin or others stained ones. This approach may also be useful in any crystal deposition induced diseases.
Keywords
Method of “not-staining”; Gout; Chondrocalcinosis; Hydroxyapatite arthritis
Introduction
Gouty arthritis induced by crystalline monosodium salt of uric acid [C5H4N4O3] (monosodium urate monohydrate – MSU), pseudogout induced by calcium pyrophosphate dihydrate [Ca2P2O7.2H2O] (CPPD) crystals (chondrocalcinosis, pyrophosphate arthropathy) and arthropathy induced by hydroxyapatite [Ca5(PO4)3(OH)] (HA) crystals (apatite rheumatism, Milwaukee syndrome) are regarded as distinct clinical entities [1-6]. Crystals accumulate in the synovial membranes, in articular hyaline and/or fibrocartilage, joint capsules, bursae, and tendon sheaths and soft tissues with or without calcification. The exact clinical and or histological diagnosis is needed (required) because of different consequences and therapy of these diseases. The shape, the size, and the birefringence of MSU, CPPD and HA crystals, furthermore their solubility in traditional fixatives (formaldehyde) and aqueous solutions (e.g., dyes) vary. Only those minerals or crystals can be detected microscopically by staining or histochemical reactions which are remaining in tissue sections after fixation and paraffin embedding [7-11]. In conventionally processed microscopic specimens empty spaces left by dissolved crystals may indicate indirectly the earlier presence of minerals or crystals, for example empty clefts by dissolved cholesterol crystals in tissues. In our earlier studies we found that crystalline deposits may disappear not only during fixation or embedding, but may dissolve also during the staining of tissues [12-16], and the simplest and most effective “staining” technique to demonstrate crystals is “notstaining” [12]. In this short communication we demonstrate the benefit of using polarized light to visualize crystals in unstained tissue sections.
Patient Population
At the National Institute of Rheumatology and at the Hospital of the Order of the Brothers of Saint John of God in Budapest surgical specimens of 101855 patients were examined histologically between 1985 and 2010, among them 146 (0.14%) patients with tophaceous gout, 15 (0.014%) with chondrocalcinosis and 3 (0.0029%) with hydroxyapatite deposits (Milwaukee syndrome). Two hundred eight (208) tissue samples of 146 patients with clinically diagnosed gout, 29 tissue samples of 15 patients with clinically diagnosed chondrocalcinosis, and 14 tissue samples of 3 patients with clinically diagnosed apatite rheumatism were studied. The tissue blocks were fixed in 8% formaldehyde solution at pH 7.6 and embedded in paraffin. Serial sections were cut and stained with haematoxylin-eosin (H-E) [17]. Unstained tissue sections were deparaffinized and traditionally mounted with Canada balsam and cover slipped. The H-E stained and unstained sections were examined under polarized light with an Olympus BX51 polarization microscope.
Results
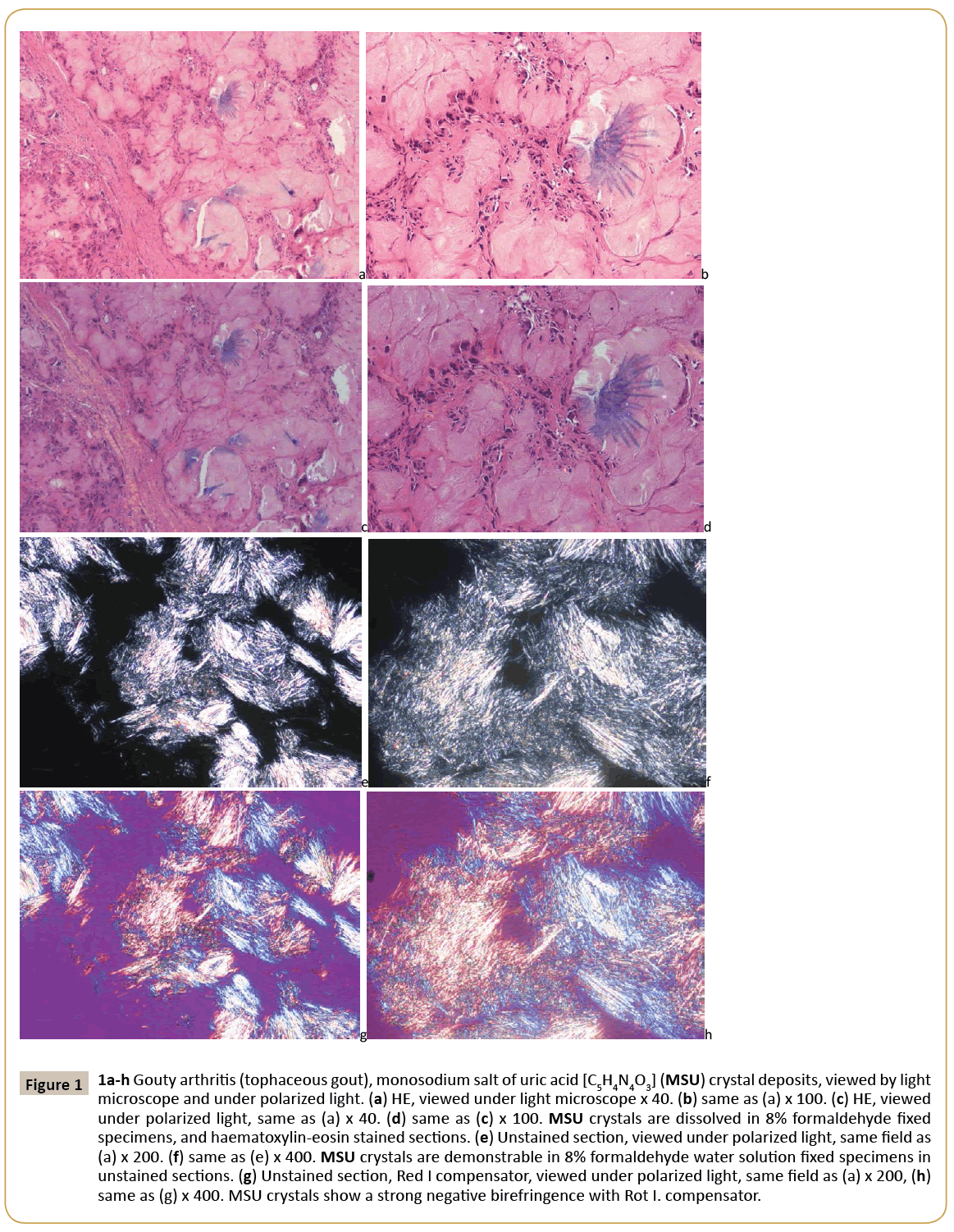
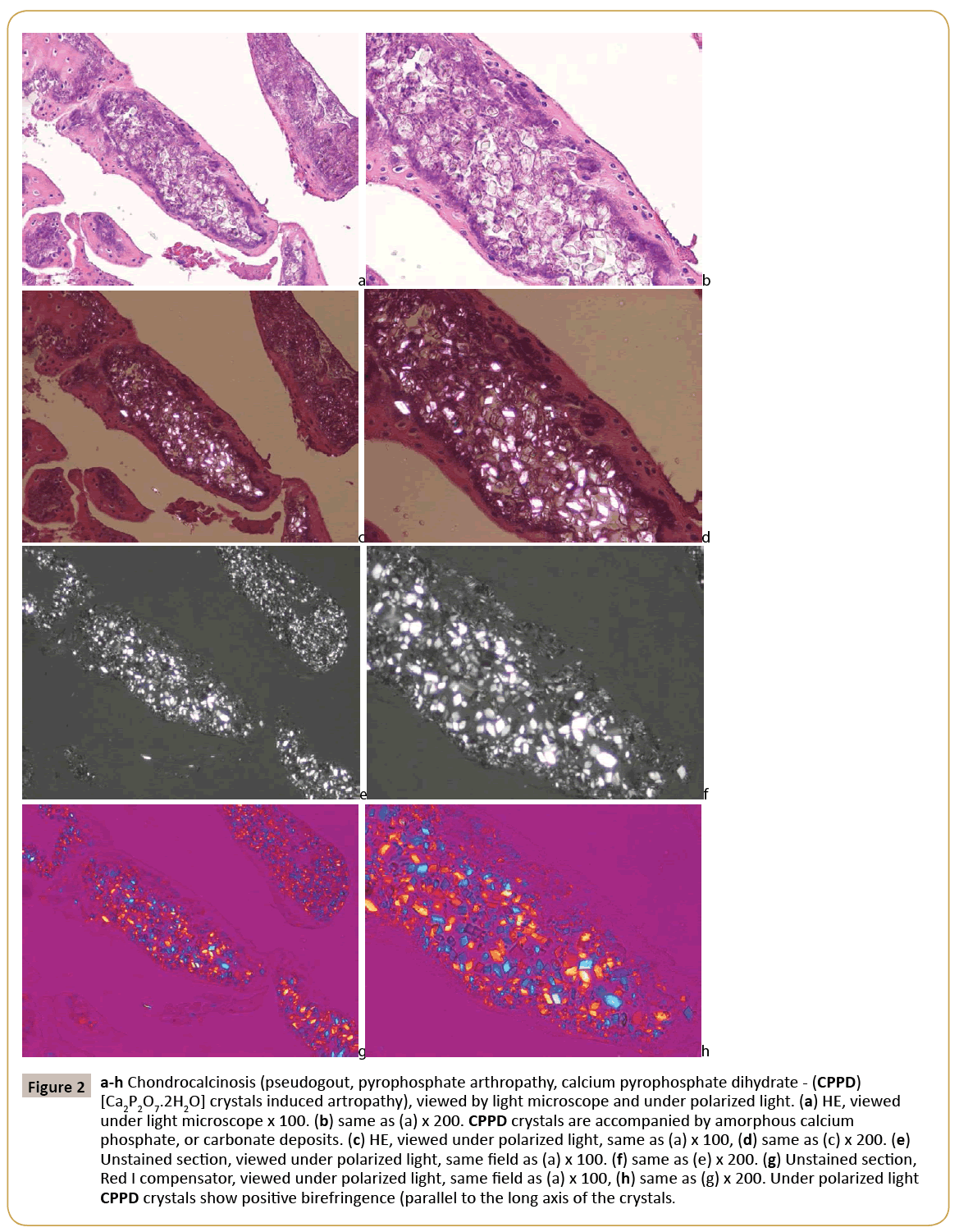
In gouty tophus the monosodium salts of uric acid (MSU) crystals were arranged typically in bundles. The sizes of the needleshaped crystals were submicroscopic- to 40 μm. Viewed under polarized light, they showed a strong negative birefringence with Rot I compensator. They were present in 30.63% of H-E stained and in 62.86% of unstained sections. The CPPD were arranged separately or in aggregates within the amorphous calcium phosphate, or carbonate deposits. The crystals were small (<40 μm), had a rhomboid shape in which adjacent sides were of unequal lengths and angles were not at 90 degrees. Viewed under polarized light, they showed a positive birefringence with Rot I compensator. They were detected in 34.48% of H-E stained and in 88.24% of unstained sections. The HA crystals are small, 50-500 nm, rod-shaped and in mineral deposits were arranged typically in 1-5 μm spheroid micro aggregates. Under polarized light the HA crystals showed a week positive birefringence with Rot I compensator; the intensity of birefringence of HA was much weaker, than the positive birefringence of CPPD crystals. They were detected in 7.14% of H-E stained and in 100.0% of unstained sections. Crystal deposits have been provoking inflammatory reaction of different intensity. Inflammatory reaction around monosodium urate deposits was much more intensive in comparison with CPPD or HA deposits. Apatite rheumatism was characterized by near the total absence of an inflammatory reaction around of HA crystal deposits. The presence of MSU, CPPD and HA crystal deposits in haematoxylin-eosin stained and in unstained tissue sections is summarized in Table 1. As evident from Table 1, certain crystals can be more frequently visualized in unstained sections of formalin fixed tissue than in H –E stained sections. The simplest method to microscopically detect crystals in crystal induced arthropathies is demonstrated in Figures 1-3.
| Crystalline deposits | n of tissue samples (Ts) n of patients (Pts) |
H-E% | Unstained% |
|---|---|---|---|
| Gout (monosodium urate - MSU) | 208 Ts of 146 Pts | 30.63 | 62.86 |
| Chondrocalcinosis (CPPD) | 29 Ts of 15 Pts | 34.48 | 88.24 |
| Hydroxyapatite arthritis (HA) | 14 Ts of 3 Pts | 7.14 | 100.0 |
Table 1: MSU, CPPD and HA crystal deposits in haematoxylin-eosin stained and in unstained tissue sections.
Figure 1: 1a-h Gouty arthritis (tophaceous gout), monosodium salt of uric acid [C5H4N4O3] (MSU) crystal deposits, viewed by light microscope and under polarized light. (a) HE, viewed under light microscope x 40. (b) same as (a) x 100. (c) HE, viewed under polarized light, same as (a) x 40. (d) same as (c) x 100. MSU crystals are dissolved in 8% formaldehyde fixed specimens, and haematoxylin-eosin stained sections. (e) Unstained section, viewed under polarized light, same field as (a) x 200. (f) same as (e) x 400. MSU crystals are demonstrable in 8% formaldehyde water solution fixed specimens in unstained sections. (g) Unstained section, Red I compensator, viewed under polarized light, same
Figure 2: a-h Chondrocalcinosis (pseudogout, pyrophosphate arthropathy, calcium pyrophosphate dihydrate - (CPPD) [Ca2P2O7.2H2O] crystals induced artropathy), viewed by light microscope and under polarized light. (a) HE, viewed under light microscope x 100. (b) same as (a) x 200. CPPD crystals are accompanied by amorphous calcium phosphate, or carbonate deposits. (c) HE, viewed under polarized light, same as (a) x 100, (d) same as (c) x 200. (e) Unstained section, viewed under polarized light, same field as (a) x 100. (f) same as (e) x 200. (g) Unstained section, Red I compensator, viewed under polarized light, same field as (a) x 100, (h) same as (g) x 200. Under polarized light CPPD crystals show positive birefringence (parallel to the long axis of the crystals.
Figure 3: a-h Hydroxyapatite arthropathy (Milwaukee syndrome, apatite rheumatism) induced by hydroxyapatite [Ca5(PO4)3(OH)] (HA) crystals, viewed by light microscope and under polarized light. (a) HE, viewed under light microscope x 100, (b) same as (a) x 200. HA crystals are small, 50-500 nm, rod-shaped and are arranged typically in 1-5 µm spheroid microaggregates. (c) HE, viewed under polarized light, same as (a) x 100, (d) same as (c) x 200. (e) Unstained section, viewed under polarized light, same field as (a) x 100. (f) same as (e) x 200. (g) Unstained section, Rot I compensator, viewed under polarized light, same field as (a) x 100. (h) same as (g) x 200. Under polarized light HA and CPPD crystals show positive birefringence, but the intensity of birefringence of HA is much weaker, than the birefringence of CPPD crystals.
Discussion
The solubility of studied crystals is different: CCPD crystals are less soluble than urate or hydroxyapatite in comparison e.g., with cholesterol. The crystals may be dissolved in tissues during fixation in formalin, embedding in paraffin or during staining. In gout most urate crystals are dissolved during the haematoxylin-eosin staining procedure [13-16]. In diagnostic pathology Alizarin Red staining [18] (specific for calcium) or the von Kossa reaction [19] (specific for phosphate and carbonate) are used to demonstrate calcium and phosphate. With these methods amorphous calcium phosphate, or carbonate deposits – accompanied by CPPD crystals – stain red, or black, resp., but CPPD crystals do not stain. The masses of amorphous calcium phosphate and carbonate may mask the crystals, without any detectable birefringence. Major textbooks of histochemistry discuss many techniques and staining methods to demonstrate the preserved crystals and mineral deposits in tissue samples, but none mention the simplest method, namely the viewing of unstained tissue sections with polarized light [7-11]. Shidham and co-workers described a nonaqueous alcoholic eosin staining method for proper evaluation of CPPD and monosodium urate crystals under polarizing microscope [20]. Our results indicate that the simplest and most effective technique to demonstrate crystal deposits in tissue samples is “not-staining” [12-16]. Theoretically the crystals may be best preserved in unstained frozen sections. For instance, cholesterol crystals may be viewed in frozen section under polarized light [21]. In frozen sections beside the huge amount of cholesterol crystal targeted crystals may be hidden. A disadvantage of using unstained sections is that parallel tissue sections have to be stained in order to study the histological appearance of the sample, since unstained sections cannot be adequately studied under the light microscope. Another disadvantage (or advantage) is that a lot of crystals may be found which differ in shape, size, arrangement or quality of birefringence from well-known crystals. Unstained frozen sections are best used when a crystal-induced or metabolic disorder is suspected. We suggest analyzing tissue specimens (viewing under polarized light) in case of suspected metabolic or crystal induced diseases (independent of alcoholic or formaldehyde fixation) in unstained tissue sections as well. Crystals remain detectable in unstained sections in the great majority of “negative” sections stained with hematoxilin-eosin [12-16].
Disclosure/Conflict of Interest
There is no conflict of interest.
References
- Wortmann RI, Kelley WN (2001) Crystal-associated synovitis. Gout and hyperuricemia. In Kelly’s Textbook of Rheumatology. 6th edn. WB Saunders Company, Philadelphia, USA, pp: 1339-1376.
- Reginato AJ, Reginato AM (2001) Diseases associated with deposition of calcium pyrophosphate or hydroxyapatite. In Kelly’s Textbook of Rheumatology. 6th edn. WB Saunders Company, Philadelphia, USA, pp: 1377-1390.
- Gardner DL, McClure J (1992) Metabolic, nutritional and endocrine diseases of connective tissue. In Pathological basis of the connective tissue diseases. 1st edn. Edward Arnold, London, Great Britain, pp: 380-407.
- McCarty DJ, Lehr RJ, Halverson PB (1983) Crystal population in human synovial fluid – identification of apatite, octoalcium phosphate, and tricalcium phosphate. Arthritis and Rheumatism 26: 1220-1224.
- Fassbender HG (2002) Crystal-associated arthropathies in Pathology and pathobiology of rheumatic diseases. 2nd edn. Springer-Verlag, Berlin, Germany, pp: 353-369.
- Mohr W (2000) Stoffwechselkrankheiten: Gicht, Kalziumpyrophophate-Arthropathie, Apatitkrankheiten. In Gelenkpathologie, historische Grundlagen, Ursachen und Entwicklungen von Gelenkleiden und ihre Pathomorphologie. 1st edn. Springer-Verlag, Berlin, Germany, pp: 181-212.
- Pearse AGE (1985) Histochemistry theoretical and applied. Churchill Livingstone, Edinburgh, London, Melbourne, New York.
- Lillie RD (1954) Histpathologic technic and practical histochemistry. The Blakiston Division McGraw-Hill Book Company, New York, Toronto, London.
- Vacca LL (1985) Laboratory manual of histochemistry. Raven Press, New York, USA.
- Carson FL (1990) Gömöri’s methenamine silver method for urates. In Histotechnology: ASCP Press, Chicago, USA, pp: 222-223.
- McManus JFA, Mowry RW (1960) Methods of general utility for the routine study of tissues. In staining methods, histologic and histochemical, Hoeber PB Inc., New York, pp: 55-72.
- Bély M, Apáthy Á (2013) Mönckeberg sclerosis – kristály indukálta angiopathia (Mönckeberg’s sclerosis: crystal-induced angiopathy). Orvosi Hetilap 154: 908-913.
- Bély M, Krutsay M (2013) A hisztokémia egy megdolni látszó dogmája – Az urát kristályok szövettani kimutathatósága. 23: 37-39.
- Bély M, Krutsay M (2013) A hisztokémia egy megdolni látszó dogmája – Az urát kristályok szövettani kimutathatósága (A tenet of histochemistry that seems to be refuted – Histologically detectable urate crystals). LAM-KID 3: 23-26.
- Bély M, Krutsay M (2013) Az urát-kristályok kimutatásának egyszeru módszere formalin fixált szövetmintákon (A simple method to detect urate crystals in formalin fixed tissue). LAM 23: 271-275.
- Bély M, Krutsay M (2013) Az elodök igazsága - Záró gondolatok az urát-kristályok oldhatóságáról-oldékonyságáról (Our predecessors were right - Closing remarks on the solubility of urate crystals in microscopic specimens). LAM-KID 23: 17-21.
- Carson FL (1990) Mayer’s hematoxilin. In Histotechnology, ASCP Press: Chicago, USA, pp: 100-103.
- Lillie RD (1954) Histpathologic technic and practical histochemistry. The Blakiston Division McGraw-Hill Book Company, New York, USA, pp: 264-265.
- Vacca LL (1985) Laboratory manual of histochemistry. Raven Press, New York, USA, pp: 333-334.
- Shidham V, Chivukula M, Basir Z, Shidham G (2001) Evaluation of crystals in formalin-fixed, paraffin-embedded tissue sections for the differential diagnosis of pseudogout, gout and tumoral calcinosis. Modern Pathology 14: 806-810.
- Balogh K (2013) Personal communication, Associate Professor of Pathology, Harvard Medical School, Harvard University, USA.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences