Crystal Structure Analysis of Epoxy Derivatives
T Sankar, Potharaju Raju, Arasambattu K Mohanakrishnan, S Naveen, NK Lokanath, K Gunasekaran
T Sankar1, Potharaju Raju2, Arasambattu K Mohanakrishnan2, S Naveen3, NK Lokanath4, K Gunasekaran1*
1Centre of Advanced Study in Crystallography and Biophysics, University of Madras, Guindy Campus, Chennai, India
2Department of Organic Chemistry, University of Madras, Guindy Campus, Chennai, India
3Institution of Excellence, University of Mysore, Manasagangotri, Mysore, India
4Department of Studies in Physics, University of Mysore, Manasagangotri, Mysore, India
- *Corresponding Author:
- K. Gunasekaran
Centre of Advanced Study in Crystallography and Biophysics, University of Madras, Guindy Campus, Chennai-600 025, India
E.mail: gunaunom@gmail.com
Abstract
The halogen atoms (F, Br and Cl) substituted in epoxy compounds which were crystallized in slow evaporation method. Crystallographic data were collected by using BRUKER SMART APEX II CCD detector diffractometer. All the three compounds were solved by direct methods and refined by F2 full matrix least squares method. Compounds I and III crystallizes in monoclinic crystal system P21/c space group, but compound II crystallize in Triclinic P? space group, respectively. The final R-factor of the three compounds 0.0479, 0.0500 and 0.0787 respectively.
https://myseoblog.blogdon.net/
https://myseoblog.blogaaja.fi/
https://myseoblog.jimdosite.com/
https://myseoblog.edublogs.org/
https://myseoblog.websites.co.in/
https://myseoblog47.wordpress.com/
https://myseoblog.waarnnnnnnbenjij.nu/
https://myseoblog.jigsy.com/
https://szeith-rhounds-kliagy.yolasite.com/
https://myseoblog-40.webselfsite.net/
https://myseoblog.mystrikingly.com/
https://myseoblog.splashthat.com/
https://myseoblog.webnode.com.tr/
https://myseoblog.odoo.com/
https://myseoblog.creatorlink.net/
https://whiteseotr1-s-site.thinkific.com/
https://myseoblog.estranky.cz/
https://65390c7d9a166.site123.me/
https://myblogseoooo.blogspot.com/
https://myseoblog.hashnode.dev/
https://whiteseotr1.wixsite.com/myseoblog
https://myseoblogg.weebly.com/
https://sites.google.com/view/myseoblogg/
https://codepen.io/myseoblog/pens/public
https://myseoblogg.livejournal.com/
https://wakelet.com/@myseoblog87204
https://www.homify.com/users/9537482/myseoblog/
https://theomnibuzz.com/author/myseoblog/
https://lessons.drawspace.com/profile/323508/myseoblog/
https://my.desktopnexus.com/myseoblog/
https://writeupcafe.com/profile/myseoblog/
https://www.pearltrees.com/myseoblog
https://www.easyfie.com/myseoblog
https://pharmahub.org/members/27544
https://www.zupyak.com/u/myseoblog/posts
https://www.metroflog.co/myseoblog
https://www.fuzia.com/fz/myseoblog-myseoblog
https://tr.pinterest.com/whiteseotr1/
https://my.getjealous.com/myseoblog
https://micro.blog/myseoblog
https://www.tumblr.com/blog/myseobloggsblog
https://hub.docker.com/u/myseoblog
https://fire.blogfree.net/?act=Profile&MID=1342100
https://myseoblog.pixnet.net/blog
https://myseoblogg.seesaa.net/
https://www.threadless.com/@myseoblog/activity
https://neocities.org/site/myseoblog
https://myseoblog.amebaownd.com/
https://teletype.in/@myseoblog
https://ubl.xml.org/users/myseoblog S6t3Bh9Gwo
https://educatorpages.com/site/myseoblog/
https://myseoblog.onlc.fr/
Keywords
Halogen atoms; Epoxides; Xenobiotic compounds
Introduction
Compounds with epoxy group are found to be useful in paints, composite formations, and development of adhesins as well as in many microelectronic applications with biphenyl-type epoxy compounds [1-3]. Epoxides are three-membered oxygen compounds, generated when endogenous as well as xenobiotic compounds undergo oxidative metabolism via chemical and enzymatic oxidation processes. The epoxides are generally unstable in aqueous environments and highly reactive. These epoxide intermediates have been implicated as potential mutagenic and carcinogenic agents [4,5]. In view of the above said properties, structural analyses of such epoxy containing compounds are carried out by many investigators. The present study explains the structural details of three epoxy derivatives.
Experimental
Synthesis of Compound I
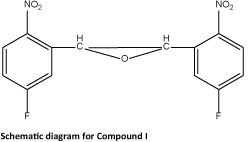
The reaction of 5-fluoro-2-nitrobenzaldehyde (0.5 g, 2.95 mmol) with triethyl phosphite (0.98 g, 5.91 mmol) in the presence of ZnBr2 (0.07 g, 0.29 mmol) at room temperature for 20 min followed by different procedures using the above mentioned general procedure gave trans-epoxide as a colorless solid. Single crystals suitable for X-ray diffraction experiments were obtained by slow evaporation of the compound in chloroform/ethyl acetate. The schematic diagram of compound I is as follows:
Synthesis of Compound II
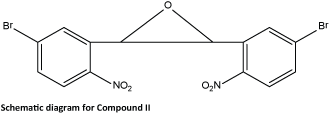
The reaction of 5-bromo-2-nitrobenzaldehyde (0.5 g, 2.17 mmol) with triethyl phosphite (0.72 g, 4.34 mmol) in the presence of ZnBr2 (0.05 g, 0.21 mmol) at room temperature for 10 min followed by different procedures using the above mentioned general procedure furnished trans-epoxide as a colorless solid. Single crystals suitable for X-ray diffraction studies were obtained by slow evaporation of the compound in chloroform/ethyl acetate. The schematic diagram of compound II is as follows:
Synthesis of Compound III
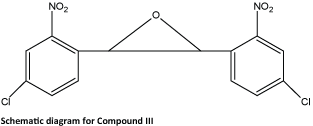
The reaction of 4-chloro-2-nitrobenzaldehyde (0.5 g, 2.69 mmol) with triethyl phosphite (0.89 g, 5.39 mmol) in the presence of ZnBr2 (0.06 g, 0.27 mmol) at room temperature for 10 min followed by different procedures using the above mentioned general procedure furnished trans-epoxide 2b as a colorless solid. Single crystals suitable for X-ray diffraction studies were obtained by slow evaporation of the compound in chloroform/ ethyl acetate. Schematic diagram of compound III is as follows:
Data collection
X-ray diffraction intensity data were collected for all three compounds on Bruker Kappa Apex II single crystal X-ray diffractometer equipped with graphite mono- chromate CuKα (λ=1.54178 Å )radiation and CCD detector. Crystals were cut to suitable size and mounted on a glass fibre using cyano acrylate adhesive. The unit cell parameters were determined from 36 frames measured (0.5° phi-scan) from three different crystallographic zones and using the method of difference vectors. The intensity data were collected with an average fourfold redundancy per reflection and optimum resolution (0.75 Å). The intensity data collection, frames integration, Lorentz and polarization correction and decay correction were done using SAINT-NT (version7.06a) software. Empirical absorption correction (multi-scan) was performed using SADABS program.
Result and Discussion
Structure solving and refinement
Crystal structure was solved by direct methods using SHELXS-97. All the non hydrogen atoms were located without any difficulty. The structure was then refined by full-matrix least-squares method using SHELXL- 97. They arrived model was refined using isotropic thermal parameters followed by anisotropic thermal parameters refinements. After completion of the refinement where R factor is converged with negligible shift/e.s.d and agreeable GooF and other parameters, hydrogen atoms were positioned geometrically C—H=0.93–0.98 Å and allowed to ride on their parent atoms, with Uiso(H) =1.5Ueq(C) for methyl H 1.2Ueq(C) for other H atoms. The relevant details of crystal data table is given in Table 1.
| Parameters | Compound I | Compound II | Compound III |
|---|---|---|---|
| Empirical formula | C14 H8 F2 N2 O5 | C14 H8 Br2 N2 O5 | C14 H8 Cl2 N2 O5 |
| Formula weight | 322.22 | 444.04 | 355.12 |
| Temperature | 296 K | 296 K | 296 K |
| Wavelength | 1.54178 Å | 1.54178 Å | 1.54178 Å |
| Crystal system, space group | Monoclinic, P21/c | Triclinic, P-1 | Monoclinic, P21/c |
| Unit cell dimensions | a = 7.8814(6)Å b = 6.4507(5)Å, β = 91.3195° c = 25.5222(2)Å |
a = 7.7239(3)Å, a=78.612(1)° b = 9.7636(3)Å, β=77.699(1)° c = 10.5752(4)Å, γ=68.967(1)° |
a = 7.1078(2) Å b = 8.0336(2) Å, β = 90.439(2)° c = 12.9753(3)Å |
| Volume | 1297.2217 Å3 | 720.86(4) Å3 | 724.3(3) Å3 |
| Z, Calculated density | 4, 1.650 Mg/m3 | 2, 2.046 Mg/m3 | 2, 1.628 Mg/m3 |
| Absorption coefficient | 1.272 mm-1 | 7.416 mm-1 | 4.311 mm-1 |
| F000 | 656 | 432 | 360 |
| Crystal size | 0.29 × 0.28 × 0.27 mm | 0.20× 0.22× 0.25 mm | 0.21× 0.23× 0.25 mm |
| Theta range for data collection | 3.46 to 65.92° | 4.90 to 64.40 ° | 6.87 to 64.19° |
| Limiting indices | -8<=h<=9, -7<=k<=7, -29<=l<=27 |
-9<=h<=8, -11<=k<=11, -12<=l<=11 |
-7<=h<=8, -9<=k<=8, -13<=l<=14 |
| Reflections collected / unique | 9298 / 2142 [Rint = 0.0652] | 7371 / 2356 [Rint = 0.0435] | 2969 / 2072 [Rint = 0.0369] |
| Completeness to theta = 65.92 | 95.10% | 97.50% | 85.5 % |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 2142 / 0 / 208 | 2356 / 0 / 208 | 2072 / 0 / 209 |
| Goodness-of-fit on F2 | 1.065 | 1.393 | 1.007 |
| Final R indices [I>2sigmaI] | R1 = 0.0479, wR2 = 0.1307 | R1 = 0.0500, wR2 = 0.1540 | R1 = 0.0787, wR2 = 0.2075 |
| R indices all data | R1 = 0.0616, wR2 = 0.1516 | R1 = 0.0502, wR2 = 0.1542 | R1 = 0.1116, wR2 = 0.2426 |
| Largest diff. peak and hole | 0.302 and -0.287 e. Å-3 | 0.932 and -1.635 e.A-3 | 0.378 and -0.434 e.A-3 |
Table 1: Crystal data for Compound I, Compound II and Compound III.
For Compound I
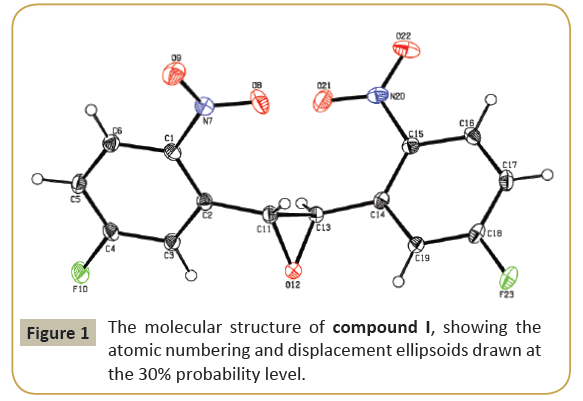
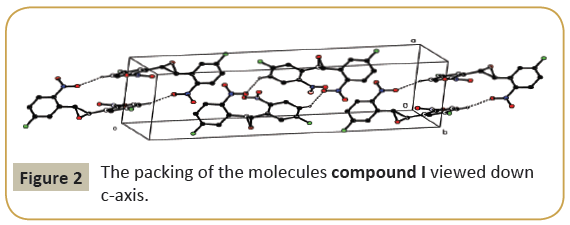
The molecular structure (ORTEP diagram) of compound I is shown in Figure 1. The bond lengths and bond angles are listed in Table 2. The epoxy ring (O12/C11/C13) plane is oriented axially with two fluro phenyl rings (C1—C6) and (C14—C19) makes dihedral angles of 66.75(19)° and 64.79\(18)°, respectively. The dihedral angle between the two fluro phenyl rings is 55.89(11)°. The nitro group (N20/O21/O22) is lie in a plane with one of the fluro phenyl ring (C14-C19/F23) which evidenced by the torsion angle values are [C14/C15/N20/O21=] 3.2(3)° and [C16/C15/ N20/O22=] 2.3(3)° and another nitro group (N7/O8/O9) is not lie in a plane with the fluro phenyl ring (C1-C6/F10). C-H…O types of intermolecular interaction makes R22(22) dimer ring motifs. C-H…O and C-H…F types hydrogen bond stabilize the crystal packing (Figure 2). Relevant hydrogen bond details are given in Table 3.
| Atoms | Length | Atoms | Angle |
|---|---|---|---|
| F23-C18 | 1.3593 | C18-C19-C14 | 119.52 |
| F10-C4 | 1.3573 | C19-C14-C15 | 116.82 |
| O12-C13 | 1.4353 | C19-C14-C13 | 118.12 |
| O12-C11 | 1.4393 | C15-C14-C13 | 125.02 |
| O21-N20 | 1.2243 | C16-C15-C14 | 122.52 |
| O9-N7 | 1.2233 | C16-C15-N20 | 116.42 |
| O22-N20 | 1.2273 | C14-C15-N20 | 121.22 |
| O8-N7 | 1.2223 | C4-C3-C2 | 119.42 |
| N7-C1 | 1.4663 | C6-C1-C2 | 122.72 |
| N20-C15 | 1.4633 | C6-C1-N7 | 117.12 |
| C19-C18 | 1.3793 | C2-C1-N7 | 120.22 |
| C15-C16 | 1.3943 | O12-C13-C11 | 59.2815 |
| C3-C4 | 1.3713 | O12-C13-C14 | 115.9519 |
| C1-C6 | 1.3793 | F10-C4-C3 | 117.62 |
| C1-C2 | 1.4063 | F10-C4-C5 | 118.52 |
| C13-C11 | 1.4743 | F23-C18-C17 | 118.52 |
| C4-C5 | 1.3774 | F23-C18-C19 | 117.42 |
| C18-C17 | 1.3724 | C17-C18-C19 | 124.12 |
| C17-C16 | 1.3824 | O12-C11-C13 | 58.9815 |
| C13-O12-C11 | 61.7415 | O12-C11-C2 | 115.72 |
| O8-N7-O9 | 123.12 | C13-C11-C2 | 121.32 |
| O8-N7-C1 | 118.72 | C18-C17-C16 | 117.22 |
| O9-N7-C1 | 118.12 | C18-C17-H17 | 121.4 |
| O21-N20-O22 | 123.52 | C16-C17-H17 | 121.4 |
| O21-N20-C15 | 118.52 | C4-C5-C6 | 117.42 |
| O22-N20-C15 | 118.12 | C17-C16-C15 | 119.92 |
Table 2: Selected bond lengths (Å) and bond angles (°) for Compound I.
| D-H…A | D-H | H…A | D…A | DHA |
|---|---|---|---|---|
| C5-H5…O21i | 0.93 | 2.48 | 3.232(3) | 138 |
| C11-H11...F23ii | 0.98 | 2.43 | 3.383(3) | 165 |
| C16-H16...O8iii | 0.93 | 2.53 | 3.206(3) | 129 |
| C19-H19…O22iv | 0.93 | 2.41 | 3.227(3) | 146 |
Table 3: Hydrogen bond interactions for Compound I [Å and =]. Symmetry codes: i) -1+x,y,z ii) -x,1/2+y,1/2-z iii) 1-x,2-y,-z and iv) 1-x,-1/2+y,1/2-z.
For Compound II
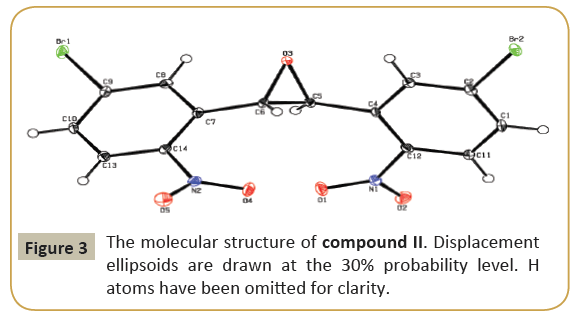
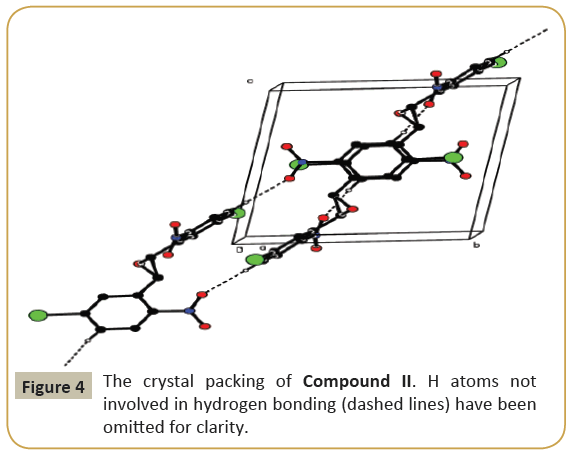
The molecular structure (ORTEP diagram) of compound II is shown in Figure 3. The bond lengths and bond angles are listed in Table 4. The epoxy ring (O3/C5/C6) plane is oriented axially with two bromo phenyl rings (C1—C4/C11/C12/Br2) and (C7—C14/ Br1) makes dihedral angles of 66.5(3)° and 70.3(3)°, respectively. The dihedral angle between the two bromo phenyl rings is 71.01(2)°. The nitro group (N1/O1/O2) is oriented with one of the bromo phenyl ring (C1-C4/C11/C12/Br2) the values of 32.0(2)° and another nitro group (N2/O4/O5) is oriented with the bromo phenyl ring (C7-C14/Br1) the values of 32.1(2)°. C-H…O types of intermolecular interaction makes R22 (22) dimer ring motifs. C-H…O types intra and inter molecular hydrogen bond stabilize the crystal packing (Figure 4). Relevant hydrogen bond details are given in Table 5 [6-12].
| Atoms | Length | Atoms | Angle |
|---|---|---|---|
| Br1-C9 | 1.889(3) | O5-N2-C14 | 118.1(3) |
| Br2-C2 | 1.892(3) | C2-C1-C11 | 118.2(3) |
| O1-N1 | 1.234(4) | C1-C2-C3 | 122.0(3) |
| O2-N1 | 1.221(4) | C1-C2-Br2 | 118.3(3) |
| O3-C5 | 1.437(4) | C3-C2-Br2 | 119.7(2) |
| O3-C6 | 1.441(4) | C2-C3-C4 | 120.4(3) |
| O4-N2 | 1.225(4) | C3-C4-C12 | 117.0(3) |
| O5-N2 | 1.233(4) | C3-C4-C5 | 120.1(3) |
| N1-C12 | 1.463(5) | C12-C4-C5 | 122.8(3) |
| N2-C14 | 1.459(5) | O3-C5-C6 | 59.1(2) |
| C1-C2 | 1.388(5) | O3-C5-C4 | 115.3(3) |
| C2-C3 | 1.390(5) | O3-C6-C5 | 58.8(2) |
| C3-C4 | 1.390(5) | O3-C6-C7 | 115.3(3) |
| C8-C9 | 1.386(5) | C10-C9-Br1 | 117.8(2) |
| C9-C10 | 1.384(5) | C8-C9-Br1 | 120.2(2) |
| C10-C13 | 1.378(5) | C13-C10-C9 | 118.5(3) |
| C11-C12 | 1.381(5) | C12-C11-C1 | 119.6(3) |
| C13-C14 | 1.392(5) | C11-C12-C4 | 122.8(3) |
| C5-O3-C6 | 62.1(2) | C11-C12-N1 | 117.0(3) |
| O2-N1-O1 | 124.4(3) | C4-C12-N1 | 120.3(3) |
| O2-N1-C12 | 118.5(3) | C10-C13-C14 | 119.9(3) |
| O1-N1-C12 | 117.1(3) | C13-C14-C7 | 122.4(3) |
| O4-N2-O5 | 124.1(3) | C13-C14-N2 | 116.9(3) |
| O4-N2-C14 | 117.8(3) | C7-C14-N2 | 120.7(3) |
Table 4: Selected bond lengths (Å) and bond angles (°) for compound III.
| D-H…A | D-H | H…A | D…A | D…H…A |
|---|---|---|---|---|
| C1-H1…O4i | 0.93 | 2.32 | 3.243(5) | 170 |
| C10-H2…O1ii | 0.93 | 2.36 | 3.286(4) | 176 |
Table 5: Hydrogen bond interactions for Compound II [Å and =]. Symmetry code: i) 2-x, -y, -z and ii) 1-x, 1-y, 1-z.
For Compound III
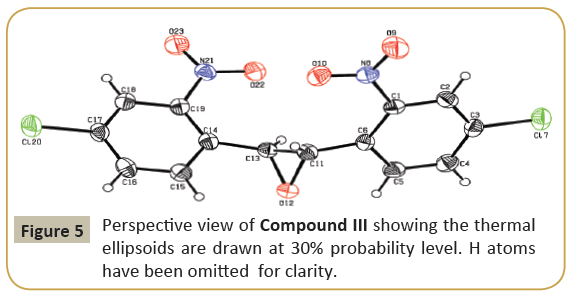
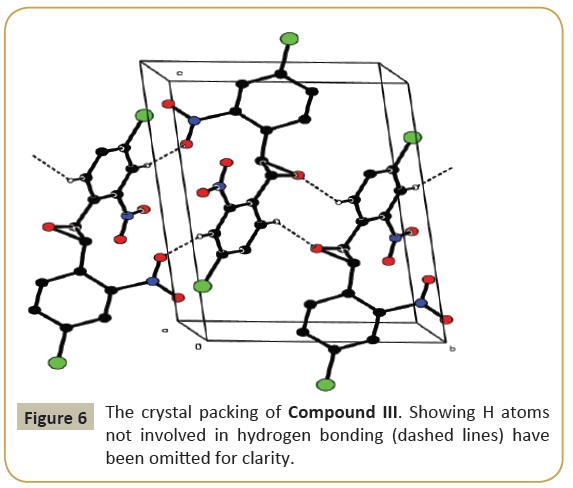
The molecular structure (ORTEP diagram) of compound III is shown in Figure 5. The bond lengths and bond angles are listed in Table 6. The epoxy ring (O12/C11/C13) plane is oriented axially with two chloro phenyl rings (C1—C6/Cl7) and (C14—C19/Cl20) makes dihedral angles of 68.6(4) and 68.0(4)°, respectively. The dihedral angle between the two chloro phenyl rings is 63.6(3)°. The nitro group (N8/O9/O10) is oriented with one of the chloro phenyl ring (C1-C6/Cl7) with the angle of 10.6(4)° and another nitro group (N21/O22/O23) is oriented with the chloro phenyl ring (C14-C19/Cl20) with the angle of 10.2(3)°. C-H…O types of intermolecular interaction makes R22 (20) and R22 (10) dimer ring motifs. C-H…O types intra and inter molecular hydrogen bond stabilize the crystal packing (Figure 6). Relevant hydrogen bond details are given in Table 7.
| Atoms | Length | Atoms | Angle |
|---|---|---|---|
| Cl7-C3 | 1.741(6) | O9-N8-C1 | 117.8(5) |
| Cl20-C17 | 1.737(6) | C2-C1-C6 | 122.8(5) |
| O12-C13 | 1.424(7) | C2-C1-N8 | 117.0(5) |
| O12-C11 | 1.435(6) | C6-C1-N8 | 120.1(5) |
| O10-N8 | 1.205(6) | C14-C19-C18 | 122.9(5) |
| O23-N21 | 1.220(7) | C14-C19-N21 | 122.1(5) |
| N21-O22 | 1.224(6) | C18-C19-N21 | 115.0(5) |
| N21-C19 | 1.475(8) | O12-C13-C11 | 59.5(3) |
| N8-O9 | 1.220(6) | O12-C13-C14 | 116.5(5) |
| N8-C1 | 1.462(7) | C11-C13-C14 | 122.4(5) |
| C6-C11 | 1.492(7) | C2-C3-Cl7 | 120.0(5) |
| C3-C2 | 1.374(8) | C4-C3-Cl7 | 119.4(4) |
| C3-C4 | 1.380(8) | C19-C14-C15 | 118.0(5) |
| C14-C15 | 1.405(8) | C19-C14-C13 | 125.8(5) |
| C18-C17 | 1.373(9) | C15-C14-C13 | 116.3(5) |
| C5-C4 | 1.372(8) | C17-C18-C19 | 117.9(6) |
| C17-C16 | 1.380(9) | C4-C5-C6 | 122.9(5) |
| C16-C15 | 1.392(8) | C1-C2-C3 | 119.2(5) |
| C13-O12-C11 | 61.7(4) | C18-C17-C16 | 121.6(5) |
| O23-N21-O22 | 122.7(5) | C18-C17-Cl20 | 119.1(5) |
| O23-N21-C19 | 119.2(5) | C16-C17-Cl20 | 119.3(5) |
| O22-N21-C19 | 118.0(5) | C5-C4-C3 | 119.0(5) |
| O10-N8-O9 | 122.6(5) | C17-C16-C15 | 119.8(6) |
| O10-N8-C1 | 119.5(4) | C16-C15-C14 | 119.8(6) |
Table 6: Bond Length and Bond Angle of Compound III.
| D-H…A | D-H | H…A | D…A | D…H…A |
|---|---|---|---|---|
| C15-H15…O12i | 0.93 | 2.57 | 3.335(8) | 140 |
| C18-H18…O10ii | 0.93 | 2.45 | 3.354(8) | 164 |
Table 7: Hydrogen bond interactions for Compound III [Å and =]. Symmetry code: i) 1-x, 2-y, 1-z and ii) 2-x, 1-y, 1-z.
Acknowledgements
The authors are thankful to Institution of Excellence, University of Mysore for providing the single-crystal X-ray diffraction facility.
References
- Lee H,Neville K (1990) In Handbook of Epoxy Resins, New York: McGraw-Hill.
- Yoda N (1997) Polym. Recent Development of Advanced Functional Polymers for Semiconductor Encapsulants of Integrated Circuit Chips and High-temperature Photoresist for Electronic Applications.AdvTechnol8: 215-226.
- Kim WG, Lee JY (2002) Polymer Grafing and Cross linking.Sci 86: 1942-1952.
- Guengrich (1982) Properties and Metabolic roles 4: 5-30.
- Sayer (1984) Stereoselectivity of Microsomal Epoxide Hydrolase toward Diol Epoxides and Tetrahydroepoxides Derived from Benz[u]anthracen.J BiolChem260: 1630-1640.
- Bruker (2008) APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Cho CS, Liau WB, Chen LW (1999) Single-crystal structure analysis of a novel aryl phosphinatediglycidyl ether.Acta Cryst55: 525-529.
- Farrugia LJ (1997) ORTEP-3 for Windows - a version of ORTEP-III with a Graphical User Interface (GUI). J Appl Cryst 30: 565.
- Flippen-Anderson JL, Gilardi R (1981)Diglycidyl ether of bisphenol A (DGEBA 37). ActaCrystallographica Section B: 1433-1435
- Adrian JF,Curtis JO(2000) Epoxide hydrolases: biochemistry and molecular biology. Chemico-Biological Interactions 129: 41-59.
- Sheldrick GM (2007)Foundations of Crystallography. Acta Cryst A 64: 112-122.
- Spek AL (2008) Structure validation in chemical crystallography. Acta Cryst D 65: 148-155.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences